Ovarian cancer is known to have the highest incidence rate among gynecological cancers in females. Most ovarian cancer cases are initially asymptomatic, and there is no proper screening method [
1,
2]. At the time of initial diagnosis, many ovarian cancer patients have already developed metastases to the local lymph nodes. The early 5-year survival rate is 90%, but the survival rate drops to as low as 20% when the tumor has metastasized to a distal site [
3]. Carboplatin, the oldest chemical compound currently used for ovarian cancer treatment, is now very important clinically for ovarian cancer patients [
4,
5]. Carboplatin has been reported to be most effective for patients with ovarian cancer [
6]. Currently, anticancer drugs such as paclitaxel, topotecan and carboplatin are used in the treatment of ovarian cancer [
7-
9]. All anticancer drugs are administered according to the patient’s diagnosis status and can be administered as a single or combined treatment [
10]. Carboplatin was developed to have fewer side effects than cisplatin and is known as an anticancer drug that significantly reduces cisplatin side effects [
11,
12]. Importantly, materials that deliver anticancer drugs to cancer cells should be nontoxic, biocompatible and biodegradable [
13-
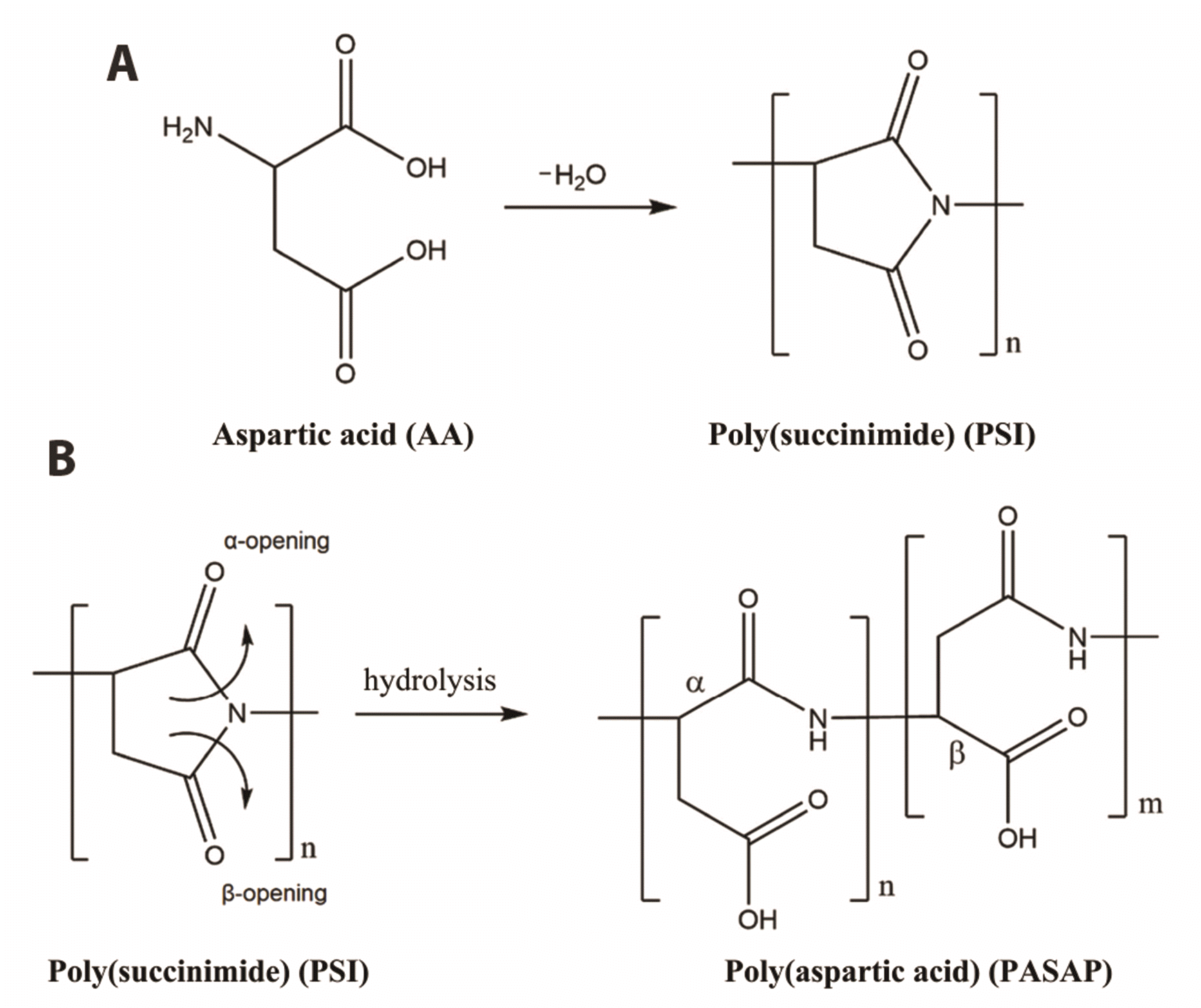
15]. The compound polysuccinimide (PSI) is a drug delivery material that is biocompatible and biodegradable and has the characteristics of and potential to be a drug conjugation material [
16-
18]. There are many ways to conjugate drugs to a delivery material, which depend on the characteristics of the material. The differences in material properties are closely related to the
in vivo pharmacokinetics after drug delivery, and the biocompatibility and biodegradability of the material should be determined by the characteristics of the delivery material used [
19]. In particular, the efficacy and effectiveness of the delivery of drugs that can alleviate certain diseases, such as cancer, are among the most important issues [
20,
21]. PSI can also be used independently, as it exhibits toxic effects to cervical and ovarian cancer cells along with antibacterial and hemolytic properties [
22]. Inducible nitric oxide synthase (iNOS) and other nitric oxide synthases promote the production of nitric oxide from L-arginine. NO is a very important molecule in cell signaling and is known to play a crucial role in many biological processes. In addition, the iNOS gene has been noted to have two functions: tumor cell suppression and tumor cell promotion [
23-
25]. In general, it has been reported that when NO is expressed at high concentrations by the actions of iNOS, it is cytotoxic and can inhibit tumor growth, while low NO expression promotes tumor growth through cell protection and cell death suppression [
26,
27]. Tumor promotion induces heat shock protein 70 and p53 mutations, inhibits T-lymphocyte proliferation by NO secretion from the tumor, and inhibits the immune response by inactivating caspases 1, 2, 3, 4, 6, 7, and 8
via S-nitrosylation [
28,
29]. All of these functions are involved in tumor promotion. Tumor suppression plays a role in tumor death
via increasing the expression level of p53 and inhibiting metastasis, cell necrosis and angiogenesis [
30]. iNOS is readily detected in a variety of ovarian cancer cell lines. However, although iNOS gene expression is often observed in ovarian cancer cells, the role of iNOS in ovarian cancer growth, survival and resistance to platinum compounds is unclear. Nonetheless, while numerous other studies have described the advantages and disadvantages of iNOS expression, these studies have not conclusively shown the direct role of iNOS in ovarian cancer cells and tumors [
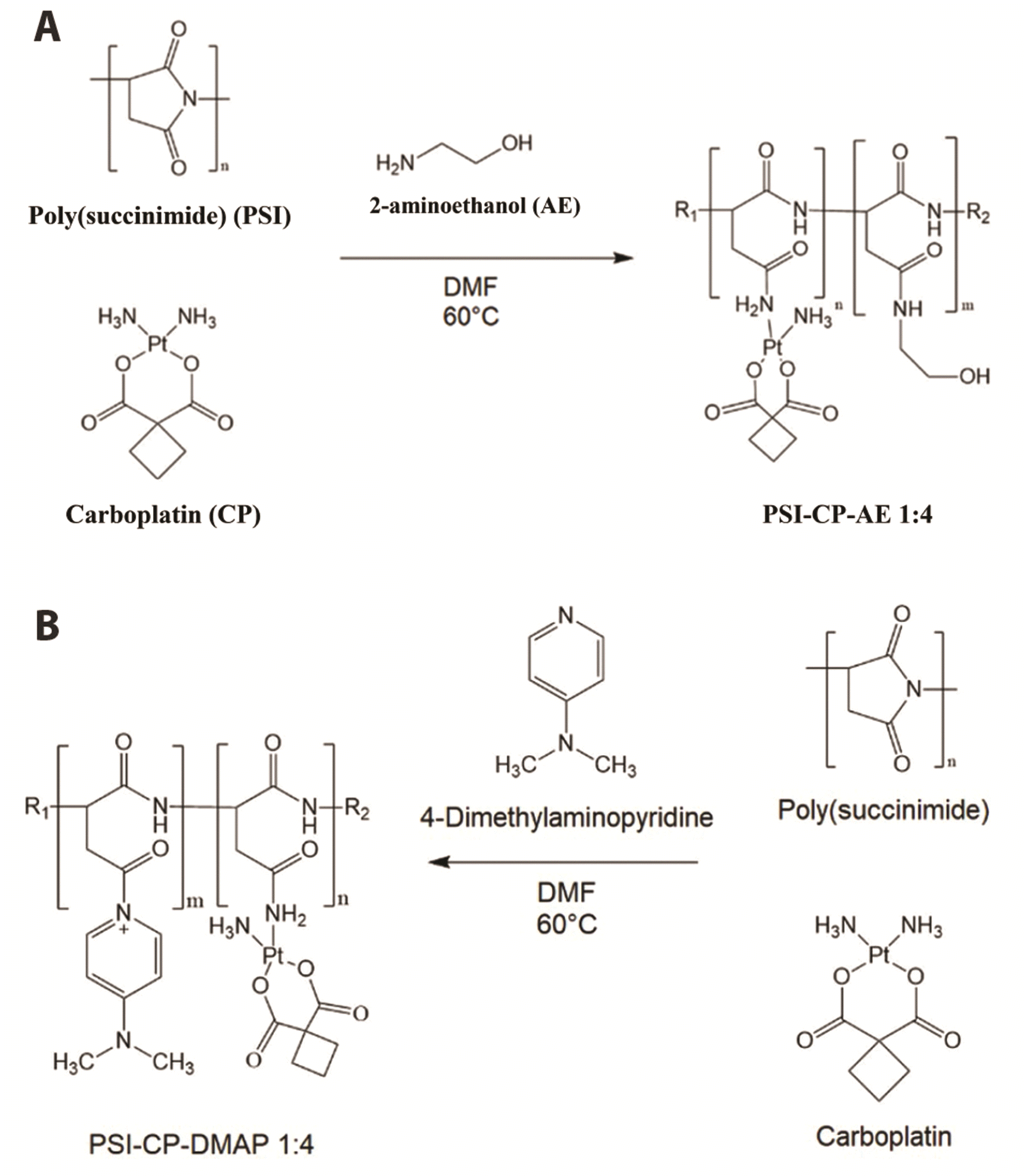
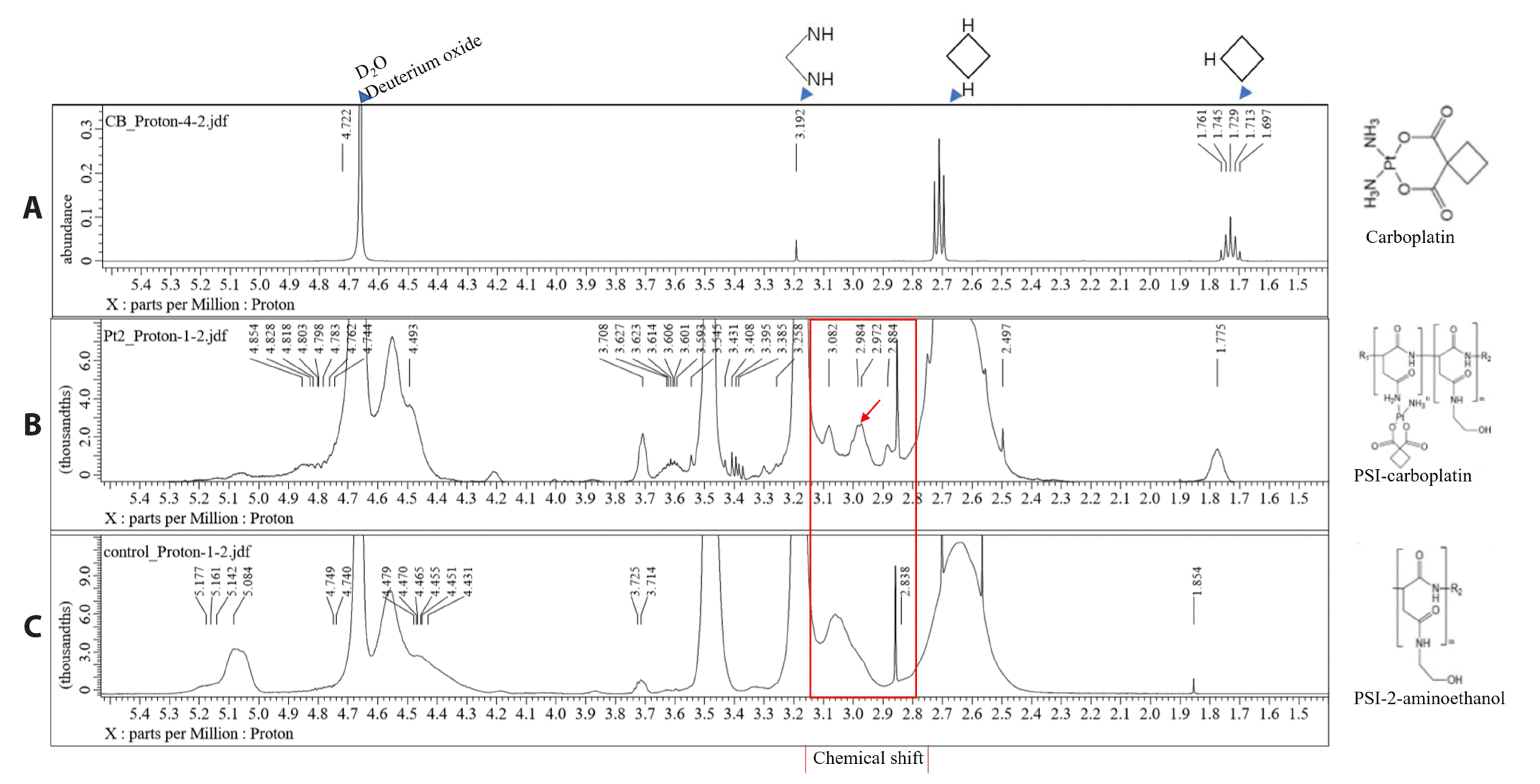
31]. In this study, we investigated the mRNA and protein expression resulting from the iNOS gene in ovarian cancer cell lines. Additionally, there have been no reports on the conjugation of PSI with platinum compounds, such as carboplatin, but conjugated carboplatin could be a candidate anticancer drug that can solve the past problem of carboplatin tolerance.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download