Abstract
Based on most neuroanatomical descriptions, the anterior spinal artery (ASA) originates from two small vessels of the vertebral arteries, which are anastomosed just before forming the basilar artery. This study aimed to determine and quantify the possible variants of the origin of the ASA and its trajectory in samples of human brain stems. Male brain stems with the superior portion of the spinal cord until myelomera C3 of 23 adult human, and no evident morphological alterations were selected. The brain stems were collected for three years and fixed in a 10% formalin solution at the Anatomy Laboratory of the Universidad de Caldas (Colombia). Five samples (21.7%) had variations in the origin and trajectory of the anterior spinal artery. The variations in the origin of the ASA could generate morphofunctional advantages instead of leading to complications. That is the case when there are two anterior spinal arteries since it would increase tissue perfusion, thus protecting part of the spinal cord from ischemic pathologies. It is essential to consider the variations that may exist in the supply of the anterior region of the spinal cord for clinical and surgical assessments due to variations in its supplied territory.
Go to : 
Based on the neuroanatomical descriptions, the anterior spinal artery (ASA) is formed by the anastomosis of two small vessels that originate from the intracranial portion of the vertebral arteries [1-4], before they anastomose to form the basilar artery. However, in anatomical dissection, the origin of the ASA is variable, being formed from several centimeters below its habitual origin, and well defined in the cervical region of the spinal cord [5]. Despite this artery’s importance in the supply of the anterior region of the spinal cord [6], there is little information in the scientific literature about the variations in its origin. It is continuously described as an artery that courses along the anterior median fissure [1] of the medulla oblongata and the spinal cord [1, 7], extending to the medullary cone. This artery supplies various structures such as the anterior funiculi, anterior horns, base of the posterior funiculi, periependymal region, and anteromedial region of the lateral funiculi. They participate in the supply of the anterior two-thirds of the spinal cord. Therefore, ischemic injury or hemorrhage of the ASA can cause ASA syndrome [8], a rare disorder usually caused by an non-trauma-related obstruction of an extravertebral artery [9]. This important spinal vessel can be injured after surgeries. However, there are differences of opinion regarding the mechanism responsible for neurological involvement [5] that would generate an alteration of blood flow to smaller-caliber vessels. The syndrome manifests motor alterations, mainly of the limbs, by affecting the corticospinal tracts that carry efferent information [10]. Although, it also affects the lateral spinothalamic tracts that transport pain and temperature stimuli, and the anterior spinothalamic tracts that carry pressure and crude touch stimuli. In these types of alterations, which may also be due to comminuted fractures of the vertebral bodies, hematic distribution is preserved to the posterior funiculi since the patient may discriminate pressure, touch, and pallesthesia [11]. This investigation aimed to determine and quantify the variants in the origin and trajectory of the ASA in a sample of human brain stems.
Go to : 
Brains with a significant portion of the brain stem and the superior portion of the spinal cord until myelomere C3 of 23 male adults were selected. The brains were collected for the last three years (2020-2022) and fixed in a 10% formalin solution at the Anatomy Laboratory of the Universidad de Caldas (Colombia). The selected blocks did not present tumors or evident defects of the gross anatomy, so the origin and course of the ASA could be studied. All the principles of the Declaration of Helsinki for medical research were followed, and the associated principles with Resolution 8430 of the Ministry of Health (Ministerio de Salud–the entity that regulates biomedical research in Colombia, 1993).
Go to : 
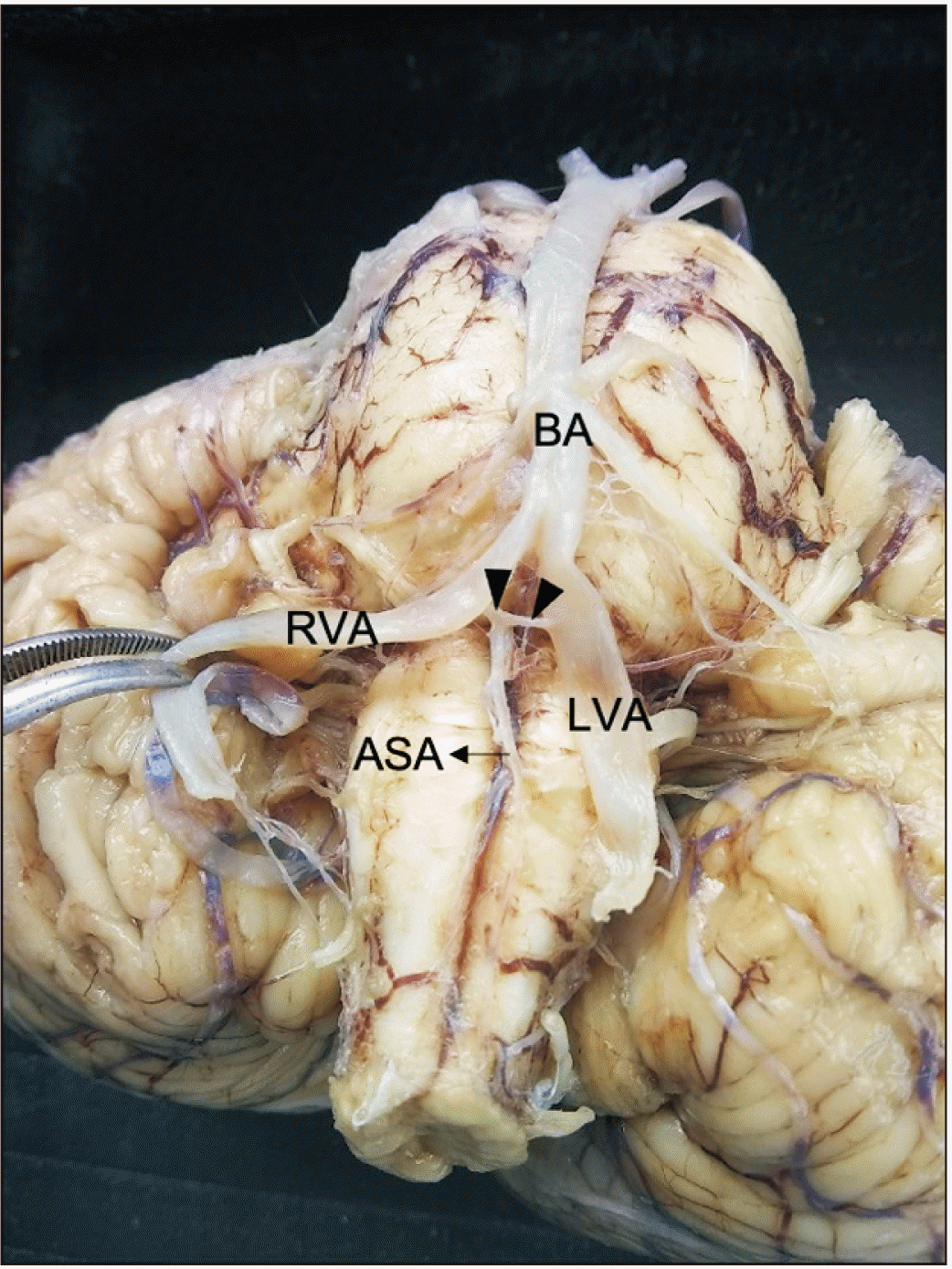
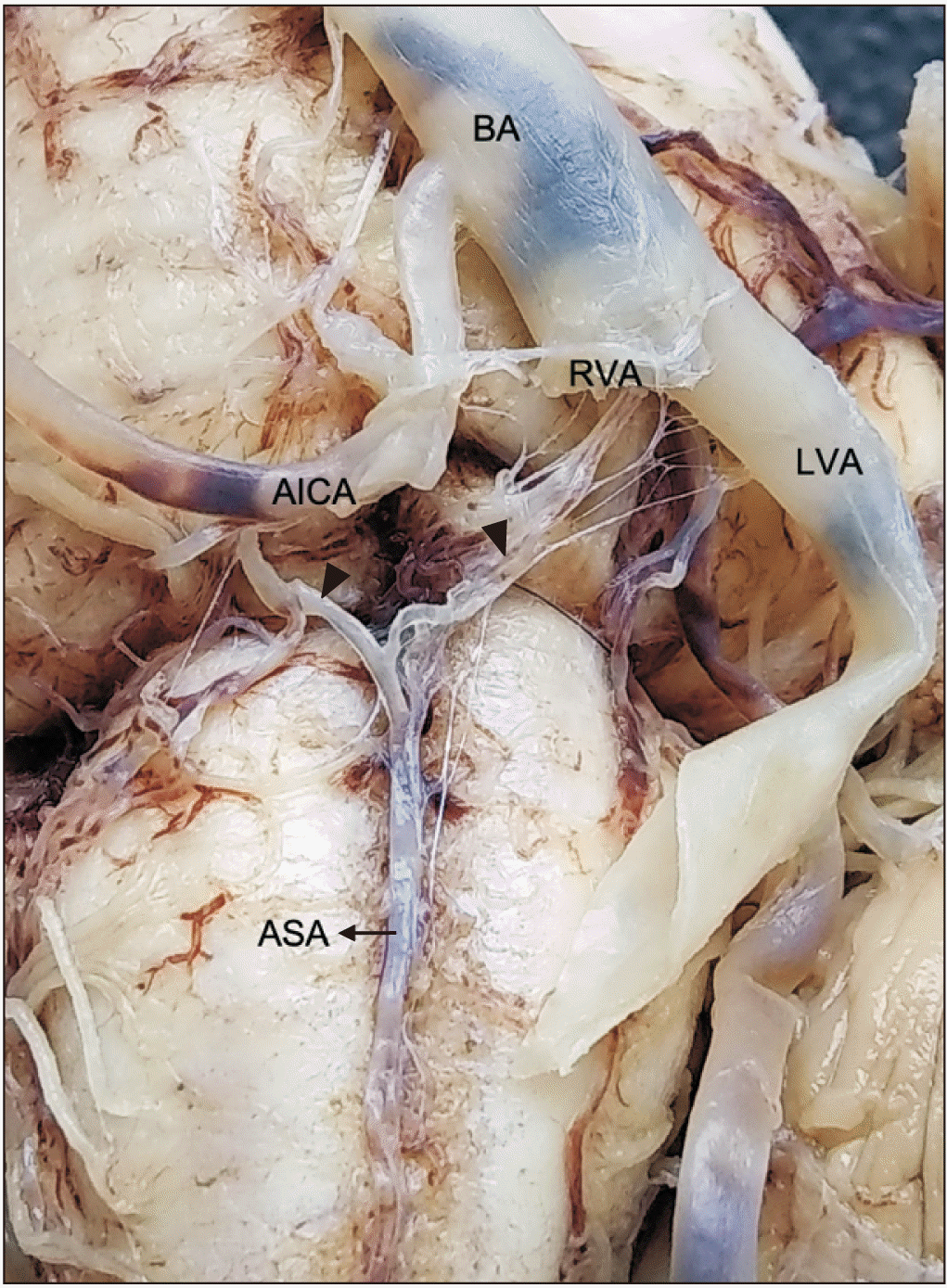
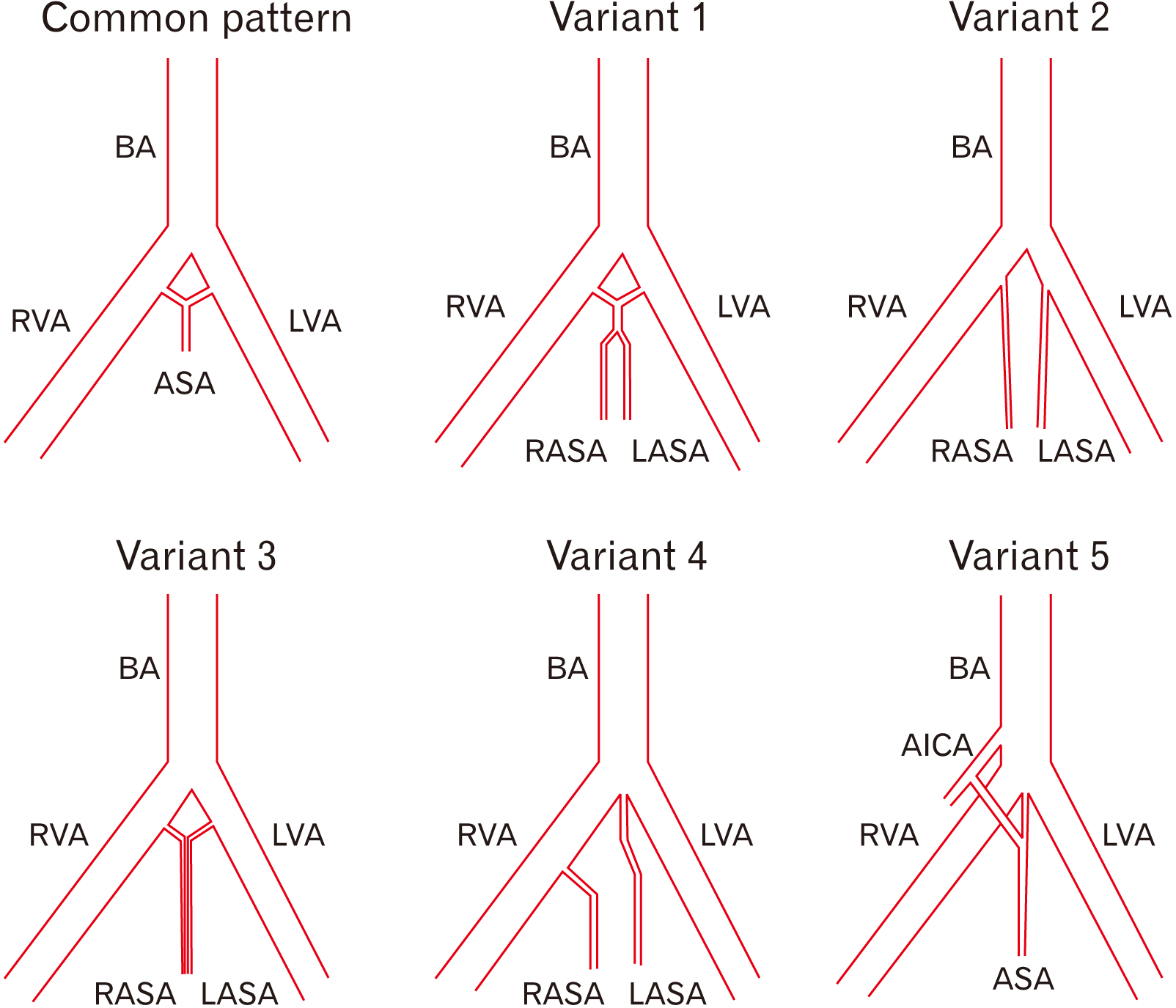
Of the 23 anatomical samples of brain stems and superior portions of the spinal cord processed in the Anatomy Laboratory at the Universidad de Caldas, 18 showed a common pattern (78.3%) (Fig. 1), and five showed anatomical variations regarding the common origin and trajectory pattern of the ASA (21.7%). In the common pattern, two small arteries of the left and right vertebral arteries anastomosed at the level of the anterior median fissure to form the ASA, which continued anteriorly to the fissure. The small arteries were named left and right prespinal arteries in the present study since both vessels are unnamed at the Terminologia Anatomica [12]. On the other side, the anatomical variations were as follows:
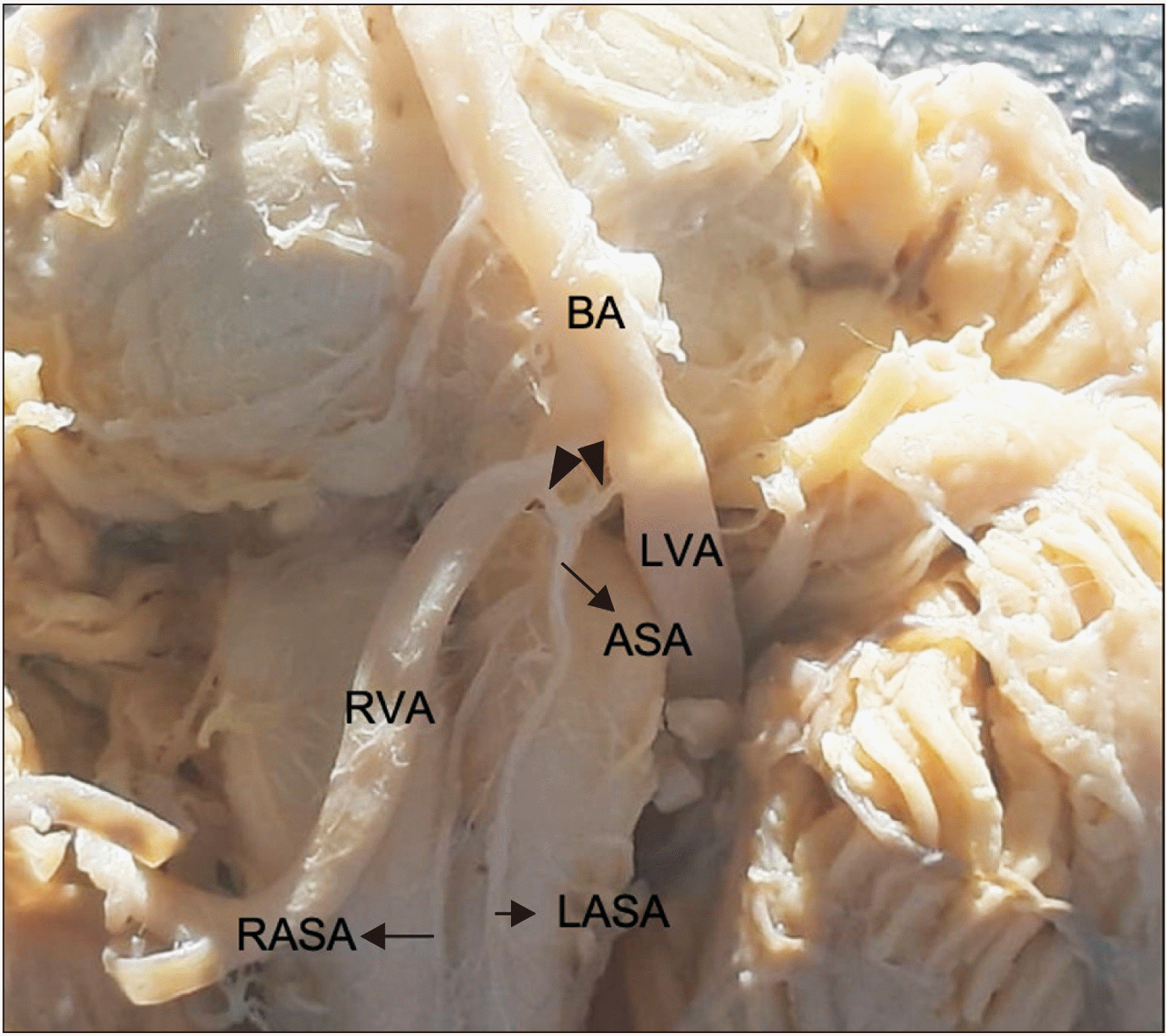
Variation 1: The left and right small prespinal arteries arose from the left and right vertebral arteries, respectively, which have a short inferior trajectory to form the ASA (the right prespinal artery was 3 mm long, and the left prespinal artery was 4 mm long). After a 9 mm course, the ASA divided into two anterior spinal arteries descending independently down the superior portion of the medulla oblongata, parallel to the pyramids and adjacent to the anterior median fissure until myelomere C3 level (4.3%) (Fig. 2).
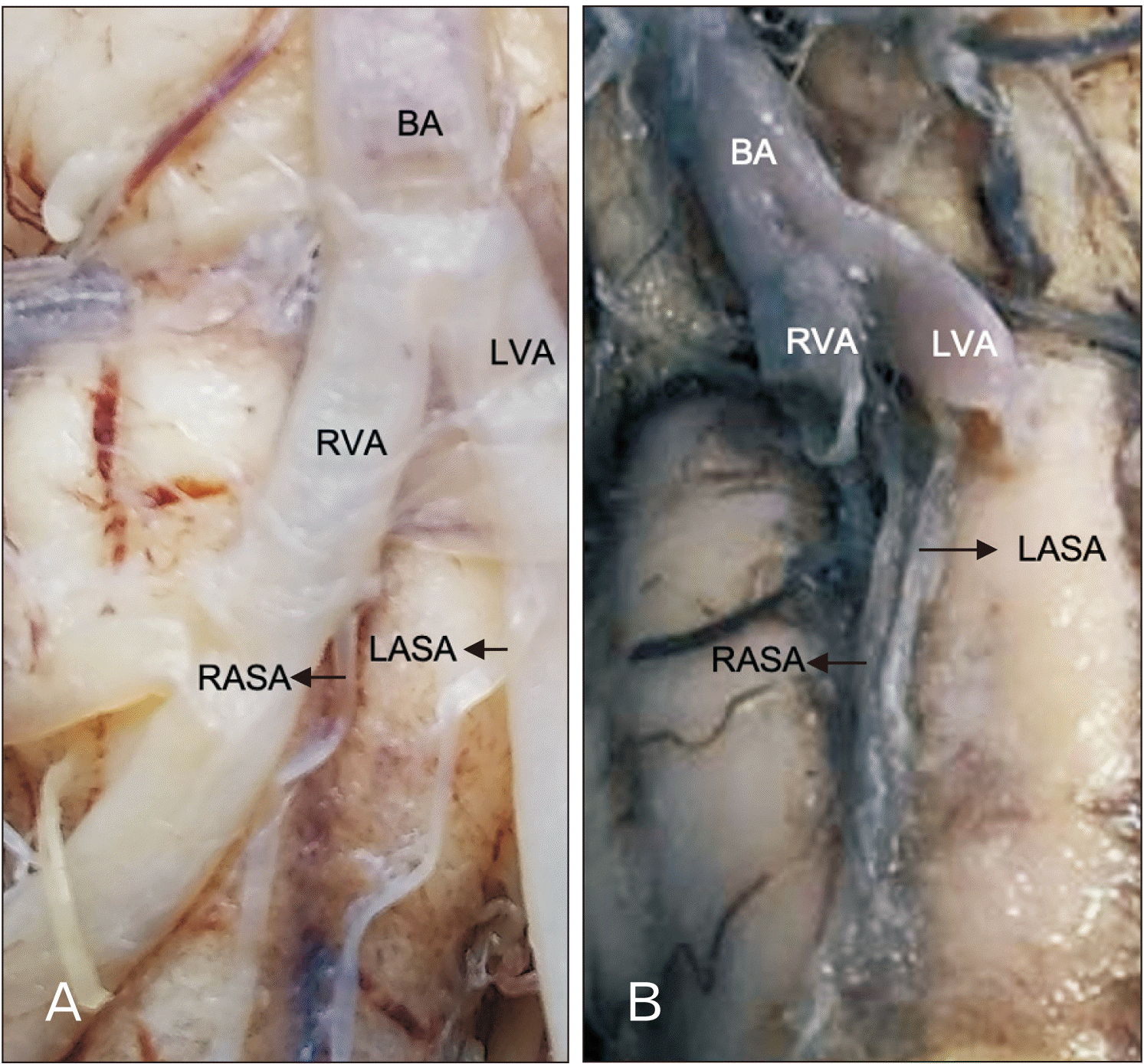
Variations 2 and 3: The ASAs arose directly from the vertebral arteries, which descend independently on each side of the anterior median fissure, adjacent to the pyramids of the medulla oblongata, with no anastomosis, until reaching myelomere C3 (4.3%) (Fig. 3A). The ASAs also arose independently from the vertebral arteries, but both descended on the anterior median fissure (4.3%) (Fig. 3B).
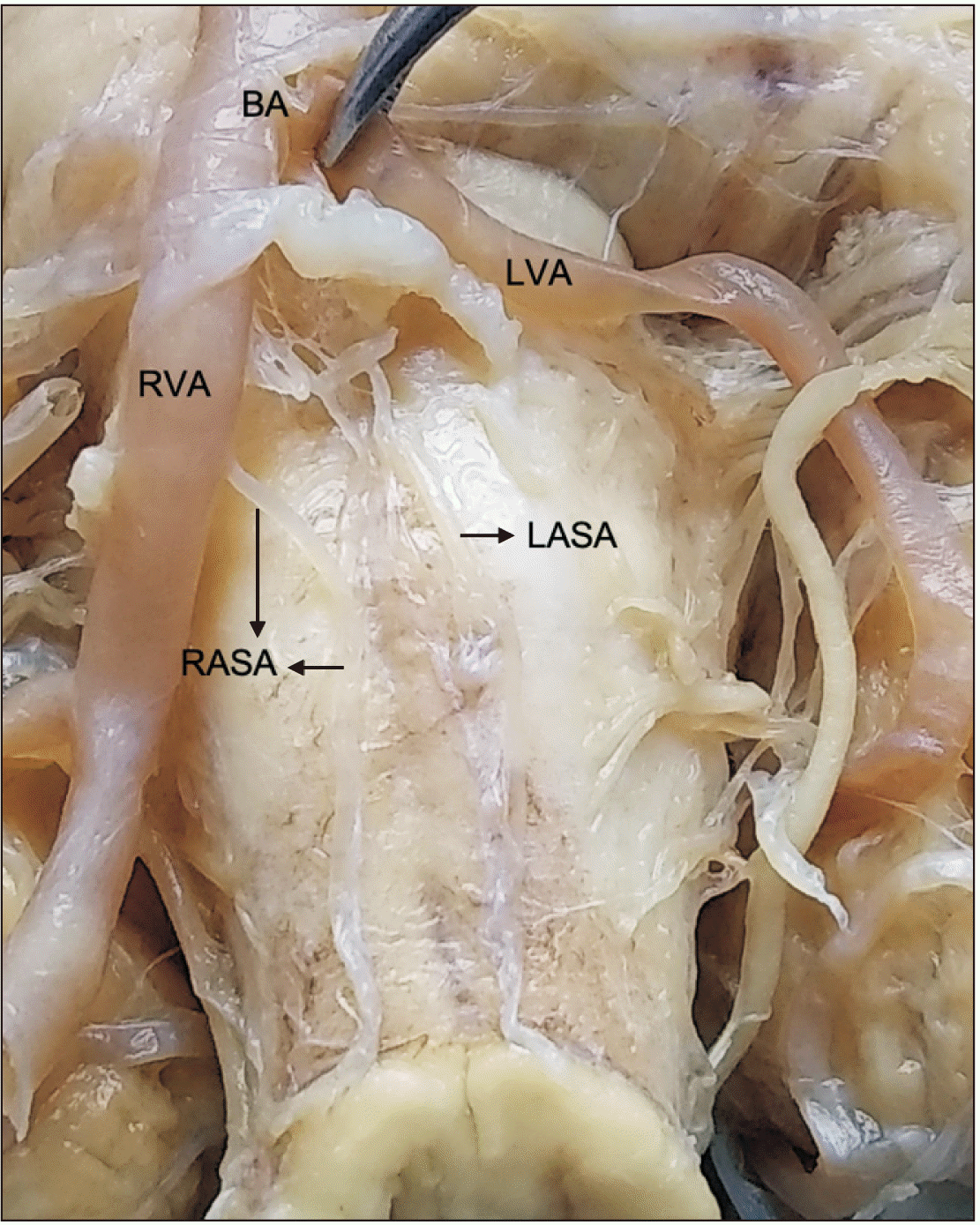
Variation 4: The left ASA arose from the point of convergence of the vertebral arteries, while the right ASA arose directly from the right vertebral artery (4.3%) (Fig. 4).
Variation 5: Finally, an ASA was formed by the convergence of a right prespinal artery formed directly from the anterior inferior cerebellar artery, and a left prespinal artery formed from the point of convergence of the vertebral arteries (4.3%). Beyond this point, the ASA followed its typical trajectory down the anterior median fissure (Fig. 5).
Based on the present study’s findings, the anatomical variations were schematized in Fig. 6.
Go to : 
Even though the pattern and site of origin of the ASA show great variability [11], as is corroborated in this paper, it is necessary to clarify that there is a discrepancy with the description that indicates that in the cases in which there are two anterior spinal arteries they anastomose to form the common anterior spinal artery, median and with no mating [6]. This paper shows that ASA can arise from the right anterior inferior cerebellar artery. In one of the specimens of the sample, there is corroboration that the origin of the two branches, right and left, that form the ASA - in other words, from the vertex of the vertebra basilar junction and the origin of the posterior inferior cerebellar artery - is very variable [11]. The absence of one of the initial anastomotic vessels that make up the ASA has been observed on several occasions, as elicited by the variations in this study. The classic vision of the supply of the anterior region of the spinal cord by an ASA is valid. However, different variations of this supply that are important for clinical and surgical assessment must be taken into account, such as has been studied in other structures of the brain as the pineal gland [13]. Since as this paper shows, two ASAs can be formed coursing down from the medulla oblongata to myelomere C3. The fact of having two anterior spinal arteries on a cervical course would imply that the causes of an injury, which include blood vessel diseases, low blood pressure with thrombosis, obstruction by tumors, intervertebral disk herniation, bone spurs, embolism [4], and bone fragment can generate a case of hemiplegia, not necessarily ipsi- and contralateral palsy. The presentation of these variations in the medulla oblongata and the superior spinal region (myelomere C3) could have implications for the formation of emboli that can run a course through a hemiside of the spinal cord and generate Brown Sequard syndrome [4] instead of a total infarction if there were a single ASA which would generate a particular morphofunctional advantage when developing these variations. Therefore, the direction of the blood flow through this spinal arterial network plays a crucial role in maintaining tissue perfusion and preventing ischemic pathology [14], contrary to what happens with a single ASA. Unfortunately, there is little information about clinical syndromes suspected to cause vascular injury of the spinal cord after thoracic surgeries, which are not rare complications, and there are discrepancies regarding the mechanism responsible for neurological involvement [5]. However, the knowledgment of these anatomical variants will allow for a better clinical approach in cases with ASA aneurysms, and although they are extremely rare, they are typically present as pseudoaneurysms [15].
Detailed knowledge of the different origin and trajectory patterns of the ASA is fundamental for surgical and endovascular procedures involving the anterior region of the spinal cord and the vertebrobasilar junction [11]. The samples processed for this study did not include the entire spinal cord. Thus, it is possible that in the specimens with two anterior spinal arteries, they merged to become a single artery somewhere below myelomere C3. The results of this study coincide with the one performed on 31 corpses [6], in which they concluded that there are always one or two anterior spinal arteries arising from the intracranial segment of the vertebral arteries.
Finally, this study shows a common origin pattern of the ASA arises from two small unnamed vessels with a value of 78.3% of the samples studied, somewhat upper than Rodríguez-Baeza et al.’s study [6] with a value of 77.4%.
In conclusion, the arteries formed by the right and left vertebral arteries that give origin to the ASA should be called right prespinal artery and left prespinal artery and be included in Terminologia Anatomica as A. prespinalis dexter and A. prespinalis sinister as anatomical variants (in parentheses) since the ASA can be formed directly from the vertebral arteries. Other studies that describe possible variants and courses of the ASA in the entire spinal cord are suggested, which would allow more explicit and more updated information on the different origin and trajectory patterns of the ASA for clinical, surgical, and endovascular procedure assessments.
Go to : 
Notes
Author Contributions
Conceptualization: JEDP. Data acquisition: JEDP, JBR. Data analysis or interpretation: JEDP, JBR, JFVG. Drafting of the manuscript: JEDP, JBR, JFVG. Critical revision of the manuscript: JEDP, JBR, JFVG. Approval of the final version of the manuscript: all authors.
Go to : 
References
1. Gövsa F, Aktan ZA, Arisoy Y, Varol T, Ozgür T. 1996; Origin of the anterior spinal artery. Surg Radiol Anat. 18:189–93. DOI: 10.1007/BF02346126. PMID: 8873332.
2. Bosmia AN, Hogan E, Loukas M, Tubbs RS, Cohen-Gadol AA. 2015; Blood supply to the human spinal cord: part I. Anatomy and hemodynamics. Clin Anat. 28:52–64. DOI: 10.1002/ca.22281. PMID: 23813725.
3. Standring S. 2008. Gray's anatomy: the anatomical basis of clinical practice. 40th ed. Churchill Livingstone;Edinburgh: DOI: 10.1002/ca.22281.
4. Duque Parra JE, Muñoz Cuervo A, Morales Parra G, Moscoso Ariza OH. 2011. Neurological anatomy with clinical orientation. Salamandra Servicios Editores;Manizales: DOI: 10.1002/ca.22281.
5. Gillilan LA. 1958; The arterial blood supply of the human spinal cord. J Comp Neurol. 110:75–103. DOI: 10.1002/cne.901100104. PMID: 13631126.
6. Rodríguez-Baeza A, Muset-Lara A, Rodríguez-Pazos M, Domenech-Mateu JM. 1989; Anterior spinal arteries. Origin and distribution in man. Acta Anat (Basel). 136:217–21. DOI: 10.1159/000146889. PMID: 2603634.
7. Biglioli P, Roberto M, Cannata A, Parolari A, Fumero A, Grillo F, Maggioni M, Coggi G, Spirito R. 2004; Upper and lower spinal cord blood supply: the continuity of the anterior spinal artery and the relevance of the lumbar arteries. J Thorac Cardiovasc Surg. 127:1188–92. DOI: 10.1016/j.jtcvs.2003.11.038. PMID: 15052221.
8. Canseco-Lima JM, Allende-Carrera R, Rodríguez-Leyva I, García-López O. 2002; Anterior spinal artery syndrome: report of a case and clinicotopographic correlation. Rev Mex Neuroci. 3:131–4.
9. Quintana-Campos JJ, Mazón-López LA, Seminario-Vergara ER, Novillo-Arévalo MB, Molina-Collantes CA, Behr-Salvador MS, Pacheco-Idrovo EP. 2020; Anterior spinal artery syndrome: clinical case report and literature review. Rev Ecuat Neurol. 29:158–60. DOI: 10.46997/revecuatneurol29100158.
10. Mancall EL, Brock DG, Gray H. 2011. Gray's clinical neuroanatomy: the anatomic basis for clinical neuroscience. Elsevier;Philadelphia:
11. Er U, Fraser K, Lanzino G. 2008; The anterior spinal artery origin: a microanatomical study. Spinal Cord. 46:45–9. DOI: 10.1038/sj.sc.3102060. PMID: 17406375.
12. FIPAT. 2017. Terminologia neuroanatomica [Internet]. The Federative International Programme for Anatomical Terminology;Halifax: Available from: https://fipat.library.dal.ca/tna/. cited 2022 Aug 6.
13. Spinelli CP, Iwanaga J, Hur MS, Dumont AS, Tubbs RS. 2022; Discovery of a trans-sellar vascular supply for the pituitary gland. Anat Cell Biol. 55:124–9. DOI: 10.5115/acb.21.255. PMID: 35599459. PMCID: PMC9256484.
14. Di Chiro G, Fried LC, Doppman JL. 1970; Experimental spinal cord angiography. Br J Radiol. 43:19–30. DOI: 10.1259/0007-1285-43-505-19. PMID: 4983587.
15. Cobb M, Griffin A, Karikari I, Gonzalez LF. 2020; Endovascular treatment of ruptured enlarging dissecting anterior spinal artery aneurysm. World Neurosurg. 139:e658–62. DOI: 10.1016/j.wneu.2020.04.100. PMID: 32339730.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download