Abstract
Solitary distal vaginal atresia is generally caused by a transverse septum or an imperforate hymen. We found a novel type of distal vaginal atresia in a late-term fetus (gestational age approximately 28 weeks) in our histology collection. This fetus had a vaginal vestibule that was closed and covered by a thick subcutaneous tissue beneath the perineal skin in the immediately inferior or superficial side of the imperforate hymen. The uterus, uterine tube, anus, and anal canal had normal development. The urethral rhabdosphincters were well-developed and had a normal topographical relationship with the vagina, but the urethrovaginal sphincter was absent. Thus, vaginal descent seemed to occur normally and form the vestibule. However, the external orifice of the urethra consisted of a highly folded duct with hypertrophied squamous epithelium. Notably, the corpus cavernosum and crus of the clitoris had poor development and were embedded in the subcutaneous tissue, distant from the vestibule. Normally, the cloacal membrane shifts from the bottom of the urogenital sinus to the inferior aspect of the thick and elongated genital tubercle after establishment of the urorectal septum. Therefore, we speculate there was a failure in the transposition of the cloacal membrane caused by decreased elongation of the genital tubercle. The histology of this anomaly strongly suggested that the hymen does not represent a part of the cloacal membrane, but is instead a product that appears during the late recanalization of the distal vagina after vaginal descent. The transverse septum was also likely to form during this recanalization.
Vaginal atresia is often associated with a duplicated uterus and unilateral renal agenesis. This condition was termed Herlyn-Werner-Wunderlich syndrome [1-5], but subsequent researchers considered this term to be a synonym of obstructed hemivagina and ipsilateral renal anomaly syndrome [6-8]. A duplicated vagina and a bicornuate uterus are the typical morphologies in these patients. In spite of the wide overlap with the aforementioned syndromes, Mayer-Rokitansky-Küster-Hauser syndrome is defined by Müllerian aplasia with vaginal atresia and a remnant uterus [9, 10]. All of these classifications are based on the classical concept of a dual developmental origin of the vagina from the Müllerian duct and the urogenital sinus. However, few surgeons have considered that an opening of the Müllerian-derived vagina descends drastically along the sinus-derived urethra [11, 12]. This descent determines not only the topographical relationship between the urethral rhabdosphincter and vagina [13, 14] but also a morphology of the vaginal vestibule.
Notably, in contrast to syndromes associated with uterus anomalies, distal vaginal atresia is likely to occur alone, without a fistula that connects the urethra or anal canal [15-18]. The solitary atresia may have an imperforate hymen and a complete or incomplete transverse septum of the vagina. Thus, it is uncertain whether the cloacal membrane or the Müllerian duct is responsible for the obstruction. Moreover, in those anomalies, there is little known about the corpus cavernosum (CC) clitoris. The drastic elongation and thickening of the tubercle change the topographical relationships between the cloacal membrane and the vaginal vestibule [19], and the tubercle has an important function in the signaling of morphogenesis in the genitalia, including the most distal urethra [20]. We believe that analysis of distal vaginal atresia during fetal development will provide the best opportunity to reanalyze the usual imperforate hymen.
The study was performed in accordance with the provisions of the Declaration of Helsinki 1995 (as revised in 2013). Our recent studies of the topographical anatomy of the fetal abdomen [21-24] examined sagittal sections from 20 late-term fetuses (gestational age [GA]: approximately 28–40 weeks, crown-rump length [CRL]: 225–328 mm). Examination of these hematoxylin and eosin (HE) stained histological sections led to the incidental finding of a rare type of vaginal atresia in a fetus with a CRL of 235 mm (GA: approximately 28 weeks). All of these fetuses were in the collection of the Department of Anatomy, Akita University (Japan), were donated by their families between 1975 and 1985, and were preserved in a 10% w/w neutral formalin solution for more than 30 years. Data on these specimens included the date of donation and the number of gestational weeks, but not the name of the family, obstetrician, hospital, reason for abortion, or genetic background. The use of these specimens for research was approved by the Akita University Ethics Committee (No. 1428).
Before using routine procedures for paraffin embedding, the fetus trunks were decalcified by incubation at room temperature in Plank-Rychlo solution (AlCl2/6H2O, 7.0 w/v%; HCl, 3.6; HCOOH, 4.6) for 3 to 7 days. Most histological images were taken with a Nikon Eclipse 80. Images at ultra-low magnification (objective lens <1×) were obtained using a high-grade flat scanner with translucent illumination (Epson scanner GTX970).
To confirm the presence of a normal vaginal vestibule at at the late phase of the vaginal descent was confirmed, because the latter process is apparently not widely known, we additionally observed sagittal histological sections from five female fetuses without vaginal atresia (GA: approximately 15–16 weeks CRL: 110–130 mm) that were part of the large collection at the Department of Anatomy of the Universidad Complutense (Madrid, Spain) were also examined. At midterm, the genital tubercle is fully developed into the cavernous tissue: the comparison in size between the midterm and late-term (the present anomaly case) seemed to be productive for discussion. These fetuses were derived from miscarriages and ectopic pregnancies from the Department of Obstetrics of the University. No information was available regarding the genetic background of the embryos or the reason for abortion. These sections were stained with HE, Azan, or Masson’s trichrome. This study was approved by the Ethics Committee of Complutense University (B08/374).
We examined the perineum and distal vagina (Figs. 1, 2) and the proximal vagina with the uterus (Fig. 3) of a late-term fetus from Akita University. This fetus had a normal urethra, but the most distal part was well below the urethral rhabdosphincter (Figs. 1A, 2A). The external orifice consisted of a highly folded duct with hypertrophied squamous epithelium (Fig. 2B). In contrast, the internal orifice at the bladder neck was normal and was surrounded by thick and smooth muscles. There was no evidence of an imperforate anus (Fig. 1A).
The anal canal was normal, was surrounded by well-developed sphincters, and had the normal transient zone of the epithelium (Figs. 1B, 2C, 3B). The posterior part of the external anal sphincter and the anal canal submucosal tissue were thicker than normal, based on our previous descriptions of late-term fetuses [25]. The anal canal had a thick longitudinal anal muscle (smooth muscle bundle) and abundant veins. The anal sinus tended to be concentrated in the anterior half of the canal, similar to our previous findings in normal fetuses [26]. There was no evidence of a candidate remnant of the tail gut below the sacrum that contained the notochord remnant in five segments. The sacrospinous ligament was well developed, and provided an origin of the gluteus maximus muscle. The coccyx was not visible in these sections.
The vagina was associated with an imperforate hymen and an abnormal vestibule (Figs. 1A, 2D, E). The imperforate hymen and vaginal wall were lined by hypertrophied squamous epithelia and tight submucosal tissues. A short longitudinal septum or fold was at the distal end of the vagina (Fig. 2D). The vaginal vestibule had a cup bottom-like space because it was closed and covered by skin and thick subcutaneous tissues (Figs. 1A, 2E). Thus, this cup attached to the socket-like end of the vagina. The anterosuperior part of the cup-like vestibule was deep and extended into the anomalous orifice of the urethra, although the posterior end was rather shallow. The vaginal vestibule had a thin recess that extended laterally (Figs. 1B, C, 2F). The tissues covering the vestibule contained smooth muscles (Fig. 2E) and cavernous tissues — the CC of the clitoris (Fig. 1A insert) and the vestibular bulb (Fig. 1C insert). This bulb, accompanied by the bulbospongiosus muscle, extended posteriorly and superficially along the lateral aspect of the cavernous tissue (Figs 1B, 2F). The urethrovaginal sphincter, previously described as a thick inferoposterior extension of the female urethral rhabdosphincter [27], appeared to be absent or replaced by subcutaneous smooth muscles.
Notably, near the abnormal orifice of the urethra (Fig. 1B), there was no evidence of a typical clitoris covered by a protrusion of the skin or epithelium in the vaginal vestibule. The CC of the clitoris was small and embedded in the subcutaneous tissue, distant from the vaginal vestibule (Fig. 1A). In spite of the absence of the glans, a prepuce-like tissue developed around the corpus (Fig. 1A insert). The crus of the clitoris was present, but appeared to be thin, it was associated with the ischiocavernosus muscle (Fig. 1B). Because of the hypertrophied epithelium and dilated lumen, the vagina was so thick that it attached to the levator ani muscle (Fig. 1C) and also to the obturator internus muscle (Fig. 3A). This fetus had a normal uterus with a cervix (Fig. 3) and an ovarian duct. A large gland was below the pubis (Fig. 3B and insert), but was much anterior to the normal location of the Bartholin gland. Consequently, the anomaly in this fetus may be summarized as a vaginal vestibule isolated by an imperforated hymen and hypertrophied genital skin containing poorly-developed cavernous tissues of the clitoris.
To show the vaginal descent as well as a normal anatomy of the genital tubercle derivatives, we examined five fetuses from Universidad Complutense that had normal pelvises, and presented the histology of one of these fetuses. In each case, a deep gulf-like vestibule of the vagina was continuous with the urethra (Fig. 4). The vagina was not yet recanalized, and merged with the urethra near the external orifice to the future vestibule (Fig. 4F, G). In each case, a long longitudinal septum was present along the future anterior wall of the vestibule (Fig. 4E). However, the hymen was not yet developed. The CC and crus of the clitoris, which was as thick as the vagina, protruded infero-anteriorly over the future vestibule (Fig. 4A–D). Notably, a large cup-like cavernous tissue (the future glans) was at the distal end of the CC.
The urethral rhabdosphincter was well developed along the anterior aspect of the distal urethra. The sphincter extended inferiorly beyond the point where the urethra and vagina met. Thus, below the pubis the inferior or distal part of the sphincter surrounded the future vestibule (urethrovaginal sphincter). The anal canal contained internal and external sphincters, as well as multiple anal sinuses. The external sphincter was thicker in the posterior side (in front of the coccyx) than in the anterior side of the anal canal.
The present pelvic anomaly, a variant of distal vaginal atresia, had three characteristic features: an imperforate hymen; a vaginal vestibule that was closed by skin and subcutaneous tissue; and no glans of the clitoris, in that there was a poorly-developed CC and crus of the clitoris without the glans, and these were distant from the vestibule. Because the fetus had a normal uterus and ovarian duct, a Müllerian duct anomaly was unlikely. This morphology is somewhat similar to fetuses described by Rea and Theron [28], which had a complete or perforated membranous septum “1 cm below” the hymen. Thus, a structure that obstructs or closes the vaginal vestibule should be distinguished from the hymen.
Irrespective of whether the hymen is perforated or imperforated, it can be classically considered to represent a part or remnant of the cloacal membrane [29]. However, according to our previous observations [13, 14], the hymen is most likely a product of a later stage of development, during recanalization of the distal vagina after vaginal descent. Therefore, our normal specimens (Fig. 4) had hymens that were underdeveloped, even though they were in midterm, long after the cloacal membrane has disappeared physiologically. The anomalous “vestibular atresia” suggested there was successful completion of vaginal descent, because the interface between the urethra and vagina was tight and normal, as described by Hinata et al. [30]. Although the folded external orifice of the urethra was not normal, this alteration seemed unrelated to the early change in location of the cloacal membrane [19], but was mostly likely caused by a cessation of growth by the genital tubercle or clitoris.
Sasaki et al. [19] concluded that a prominent ventral protrusion of the genital tubercle is necessary for the topographical change between the cloacal membrane and the urogenital sinus. This early process also seemed to occur in the present anomaly. Sasaki et al. [19] also found many apoptotic cells in the ventral or superficial side of the genital tubercle in mouse embryos at 12.25 days. The clitoris of our anomalous fetus might have failed to enter the apoptotic phase of the epithelium, resulting in a closing of the vestibule. Several recent studies demonstrated that multiple signaling pathways organize development of the genital tubercle and urethra [20, 31-33]. However, a failure or redundancy of these signaling pathways seems an unlikely cause of the underdeveloped clitoris.
Because the anomaly had well-developed walls of the anal canal, the urorectal septum had normal development. The classical view is that a superior fold reaches the cloacal membrane and divides the cloaca, and two lateral folds then unite with the superior fold to form a complete septum [34, 35]. However, some researchers denied the active descent of the superior fold or the fusion with lateral folds [36, 37]. Regardless of the mechanisms responsible for development of the urorectal septum, the present anomaly indicated that an abnormal development of the genital tubercle was unlikely to provide an anorectal anomaly. The limited abnormality might be an external anal sphincter that is thicker than normal in the posterior half, but this thickening is likely in adults [38, 39].
Recent diagnoses of cloacal malformations using ultrasound or magnetic resonance imaging demonstrated cavernous tissue and a urethral rhabdosphincter in late-term fetuses and infants [40, 41], although the radiologists and surgeons appeared uninterested in these structures. We therefore emphasize two perspectives regarding normal perineal imaging results: first, there is restriction of the female rhabdosphincter in the anterior aspect of the urethra, in contrast to the absent sphincter in the posterior aspect adjacent to the vagina; second there is a large cavernous tissue immediately below the pubis. In both genders, the CC, which is as thick as half of the pubis, is never embedded in the subcutaneous tissue.
Notes
References
1. Herlyn U, Werner H. 1971; Simultaneous occurrence of an open Gartner-duct cyst, a homolateral aplasia of the kidney and a double uterus as a typical syndrome of abnormalities. Geburtshilfe Frauenheilkd. 31:340–7. German. PMID: 5573697.
2. Wunderlich M. 1976; Unusual form of genital malformation with aplasia of the right kidney. Zentralbl Gynakol. 98:559–62. German. PMID: 936822.
3. Gazárek F, Kudela M, Zenisek L, Nevrla F. 1979; Herlyn-Werner and Wunderlich syndromes (author's transl). Zentralbl Gynakol. 101:1411–5. German. PMID: 549407.
4. Karagozov I. 1983; Herlyn-Werner-Wunderlich syndrome. Akush Ginekol (Sofiia). 22:70–6. Bulgarian. PMID: 6881475.
5. Zhang H, Qu H, Ning G, Cheng B, Jia F, Li X, Chen X. 2017; MRI in the evaluation of obstructive reproductive tract anomalies in paediatric patients. Clin Radiol. 72:612.e7–15. DOI: 10.1016/j.crad.2017.02.002. PMID: 28283284.
6. Smith NA, Laufer MR. 2007; Obstructed hemivagina and ipsilateral renal anomaly (OHVIRA) syndrome: management and follow-up. Fertil Steril. 87:918–22. DOI: 10.1016/j.fertnstert.2006.11.015. PMID: 17320871.
7. Tanitame K, Tanitame N, Urayama S, Ohtsu K. 2021; Congenital anomalies causing hemato/hydrocolpos: imaging findings, treatments, and outcomes. Jpn J Radiol. 39:733–40. DOI: 10.1007/s11604-021-01115-7. PMID: 33840015. PMCID: PMC8338850.
8. Feng T, Rao X, Yang X, Yu X, Xia F, Du X. 2022; A rare variant of obstructed hemivagina and ipsilateral renal agenesis and its improvement of classification. J Obstet Gynaecol Res. 48:869–74. DOI: 10.1111/jog.15148. PMID: 35014127.
9. Wester T, Tovar JA, Rintala RJ. 2012; Vaginal agenesis or distal vaginal atresia associated with anorectal malformations. J Pediatr Surg. 47:571–6. DOI: 10.1016/j.jpedsurg.2011.09.040. PMID: 22424355.
10. Pandya KA, Koga H, Okawada M, Coran AG, Yamataka A, Teitelbaum DH. 2015; Vaginal anomalies and atresia associated with imperforate anus: diagnosis and surgical management. J Pediatr Surg. 50:431–7. DOI: 10.1016/j.jpedsurg.2014.07.010. PMID: 25746703.
11. Shapiro E, Huang HY, Wu XR. 2000; Uroplakin and androgen receptor expression in the human fetal genital tract: insights into the development of the vagina. J Urol. 164(3 Pt 2):1048–51. DOI: 10.1016/S0022-5347(05)67247-3. PMID: 10958738.
12. van der Putte SC. 2005; The development of the perineum in the human. A comprehensive histological study with a special reference to the role of the stromal components. Adv Anat Embryol Cell Biol. 177:1–131. DOI: 10.1007/b138055. PMID: 15615037.
13. Masumoto H, Rodríguez-Vázquez JF, Verdugo-López S, Murakami G, Matsubara A. 2011; Fetal topographical anatomy of the female urethra and descending vagina: a histological study of the early human fetal urethra. Ann Anat. 193:500–8. DOI: 10.1016/j.aanat.2011.03.010. PMID: 21561749.
14. Masumoto H, Takenaka A, Rodríguez-Vázquez JF, Murakami G, Matsubara A. 2012; Reappraisal of intergender differences in the urethral striated sphincter explains why a completely circular arrangement is difficult in females: a histological study using human fetuses. Anat Cell Biol. 45:79–85. DOI: 10.5115/acb.2012.45.2.79. PMID: 22822461. PMCID: PMC3398178.
15. Acién P, Acién MI. 2011; The history of female genital tract malformation classifications and proposal of an updated system. Hum Reprod Update. 17:693–705. DOI: 10.1093/humupd/dmr021. PMID: 21727142.
16. Ruggeri G, Gargano T, Antonellini C, Carlini V, Randi B, Destro F, Lima M. 2012; Vaginal malformations: a proposed classification based on embryological, anatomical and clinical criteria and their surgical management (an analysis of 167 cases). Pediatr Surg Int. 28:797–803. DOI: 10.1007/s00383-012-3121-7. PMID: 22806600.
17. Bischoff A, Alaniz VI, Trecartin A, Peña A. 2019; Vaginal reconstruction for distal vaginal atresia without anorectal malformation: is the approach different? Pediatr Surg Int. 35:963–6. DOI: 10.1007/s00383-019-04512-2. PMID: 31256298.
18. Darwish AM. 2021; A novel technique for the reconstructive formation of an annular hymen in cases of postpubertal imperforate hymen. Sultan Qaboos Univ Med J. 21:e110–5. DOI: 10.18295/squmj.2021.21.01.015. PMID: 33777431. PMCID: PMC7968913.
19. Sasaki C, Yamaguchi K, Akita K. 2004; Spatiotemporal distribution of apoptosis during normal cloacal development in mice. Anat Rec A Discov Mol Cell Evol Biol. 279:761–7. DOI: 10.1002/ar.a.20062. PMID: 15278947.
20. Harada M, Omori A, Nakahara C, Nakagata N, Akita K, Yamada G. 2015; Tissue-specific roles of FGF signaling in external genitalia development. Dev Dyn. 244:759–73. DOI: 10.1002/dvdy.24277. PMID: 25820239.
21. Suzuki D, Kim JH, Shibata S, Murakami G, Rodríguez-Vázquez JF. 2019; Topographical anatomy of the greater omentum and transverse mesocolon: a study using human fetuses. Anat Cell Biol. 52:443–54. DOI: 10.5115/acb.19.112. PMID: 31949984. PMCID: PMC6952700.
22. Xu D, Jin ZW, Kim JH, Rodríguez-Vázquez JF, Murakami G, Hayashi S. 2020; Umbilicus and the rectus sheath: a study using human fetuses. Surg Radiol Anat. 42:461–71. DOI: 10.1007/s00276-019-02398-2. PMID: 31897654.
23. Kim JH, Hayashi S, Jin ZW, Murakami G, Rodríguez-Vázquez JF. 2020; Variations in laminar arrangements of the mesocolon and retropancreatic fascia: a histological study using human fetuses near term. Tokai J Exp Clin Med. 45:214–23. PMID: 33300593.
24. Kim JH, Hayashi S, Shibata S, Murakami G, Rodríguez-Vázquez JF. 2020; Abnormal intestinal anatomy in late-stage human fetuses: three case series. Tokai J Exp Clin Med. 45:162–9. PMID: 33300585.
25. Jin ZW, Jin Y, Li XW, Murakami G, Rodríguez-Vázquez JF, Wilting J. 2016; Perineal raphe with special reference to its extension to the anus: a histological study using human fetuses. Anat Cell Biol. 49:116–24. DOI: 10.5115/acb.2016.49.2.116. PMID: 27382513. PMCID: PMC4927426.
26. Arakawa T, Hwang SE, Kim JH, Wilting J, Rodríguez-Vázquez JF, Murakami G, Hwang HP, Cho BH. 2016; Fetal growth of the anal sinus and sphincters, especially in relation to anal anomalies. Int J Colorectal Dis. 31:493–502. DOI: 10.1007/s00384-015-2455-8. PMID: 26615552.
27. Kurihara M, Murakami G, Kajiwara M, Taguchi K, Tsukamoto T, Usui T. 2004; Lack of the complete circular rhabdosphincter and a distinct circular smooth muscle layer around the proximal urethra in elderly Japanese women: an anatomical study. Int Urogynecol J Pelvic Floor Dysfunct. 15:85–94. discussion 94DOI: 10.1007/s00192-003-1107-7. PMID: 15014934.
28. Rea E, Theron HF. 1957; Hydrometrocolpos. S Afr Med J. 31:1013–5. DOI: 10.4103/0189-6725.41637. PMID: 13495641.
29. Kanagasuntheram R, Dassanayake AG. 1958; Nature of the obstructing membrane in primary cryptomenorrhoea. J Obstet Gynaecol Br Emp. 65:487–92. DOI: 10.1111/j.1471-0528.1958.tb08546.x. PMID: 13564307.
30. Hinata N, Murakami G, Abe S, Honda M, Isoyama T, Sejima T, Takenaka A. 2012; Detailed histological investigation of the female urethra: application to radical cystectomy. J Urol. 187:451–6. DOI: 10.1016/j.juro.2011.10.037. PMID: 22177162.
31. Mo R, Kim JH, Zhang J, Chiang C, Hui CC, Kim PC. 2001; Anorectal malformations caused by defects in sonic hedgehog signaling. Am J Pathol. 159:765–74. DOI: 10.1016/S0002-9440(10)61747-6. PMID: 11485934. PMCID: PMC1850556.
32. van de Ven C, Bialecka M, Neijts R, Young T, Rowland JE, Stringer EJ, Van Rooijen C, Meijlink F, Nóvoa A, Freund JN, Mallo M, Beck F, Deschamps J. 2011; Concerted involvement of Cdx/Hox genes and Wnt signaling in morphogenesis of the caudal neural tube and cloacal derivatives from the posterior growth zone. Development. 138:3451–62. Erratum in: Development 2011;138:3859. DOI: 10.1242/dev.066118. PMID: 21752936.
33. Wang C, Gargollo P, Guo C, Tang T, Mingin G, Sun Y, Li X. 2011; Six1 and Eya1 are critical regulators of peri-cloacal mesenchymal progenitors during genitourinary tract development. Dev Biol. 360:186–94. DOI: 10.1016/j.ydbio.2011.09.020. PMID: 21968101. PMCID: PMC3225897.
34. Pohlman AG. 1911; The development of the cloaca in human embryos. Am J Anat. 12:1–26. DOI: 10.1002/aja.1000120102.
35. de Vries PA, Friedland GW. 1974; The staged sequential development of the anus and rectum in human embryos and fetuses. J Pediatr Surg. 9:755–69. DOI: 10.1016/0022-3468(74)90115-8. PMID: 4424274.
36. van der Putte SC. 1986; Normal and abnormal development of the anorectum. J Pediatr Surg. 21:434–40. DOI: 10.1016/S0022-3468(86)80515-2. PMID: 3712197.
37. Nievelstein RA, van der Werff JF, Verbeek FJ, Valk J, Vermeij-Keers C. 1998; Normal and abnormal embryonic development of the anorectum in human embryos. Teratology. 57:70–8. DOI: 10.1002/(SICI)1096-9926(199802)57:2<70::AID-TERA5>3.0.CO;2-A. PMID: 9562679.
38. Kinugasa Y, Arakawa T, Abe S, Ohtsuka A, Suzuki D, Murakami G, Fujimiya M, Sugihara K. 2011; Anatomical reevaluation of the anococcygeal ligament and its surgical relevance. Dis Colon Rectum. 54:232–7. DOI: 10.1007/DCR.0b013e318202388f. PMID: 21228674.
39. Jin ZW, Cho KH, Jang HS, Murakami G, Rodríguez-Vázquez JF, Yamamoto M, Abe SI. 2017; Coccygeal body revisited: an immunohistochemical study using donated elderly cadavers. Anat Rec (Hoboken). 300:1826–37. DOI: 10.1002/ar.23615. PMID: 28545163.
40. Dannull KA, Browne LP, Meyers MZ. 2019; The spectrum of cloacal malformations: how to differentiate each entity prenatally with fetal MRI. Pediatr Radiol. 49:387–98. Erratum in: Pediatr Radiol 2019;49:839. DOI: 10.1007/s00247-018-4302-x. PMID: 30547222.
41. Bruzeau AH, Moriau D, Bahans C, Mounayer C, Spampinato G, Guigonis V, Ballouhey Q, Fourcade L. 2021; Perineal ultrasound in infants with anteriorly displaced anus: a new decision-making tool for the surgeon? Eur J Radiol. 142:109854. DOI: 10.1016/j.ejrad.2021.109854. PMID: 34303148.
Fig. 1
Topographical anatomy of vaginal atresia in a fetus with a CRL of 235 mm (sagittal sections). (A) is an almost-midsagittal section, and the five inner squares appear at higher magnification in Fig. 2A–E; (C) is the most lateral plane and the inner square appears at higher magnification in Fig. 2F. Inserts on the left of each figure show poorly developed cavernous tissues: the CC of the clitoris, which is surrounded by the developing prepuce (top); the crus of the clitoris (middle); and the vestibular bulb (bottom). Arrows in (B) and (C) indicate an end part of a lateral recess of the closed vestibule. The clitoris is embedded in a thick subcutaneous tissue that covers and closes the vestibule, and the urethra and vagina open to the vestibule. The EAS, IAS are normal. Asterisks in (A) indicate an artifact space from the histological procedure. (A–C) were at the same magnification and the inserts were at the same magnification. Scale bar: (C) 5 mm; (bottom insert) 1 mm. CRL, crown-rump length; CC, corpus cavernosum; EAS, external anal sphincter; IAS, internal anal sphincter; BSM, bulbospongiosus muscle; GMX, gluteus maximus muscle; ICM, ischiocavernosus muscle; LAM, longitudinal anal muscle.
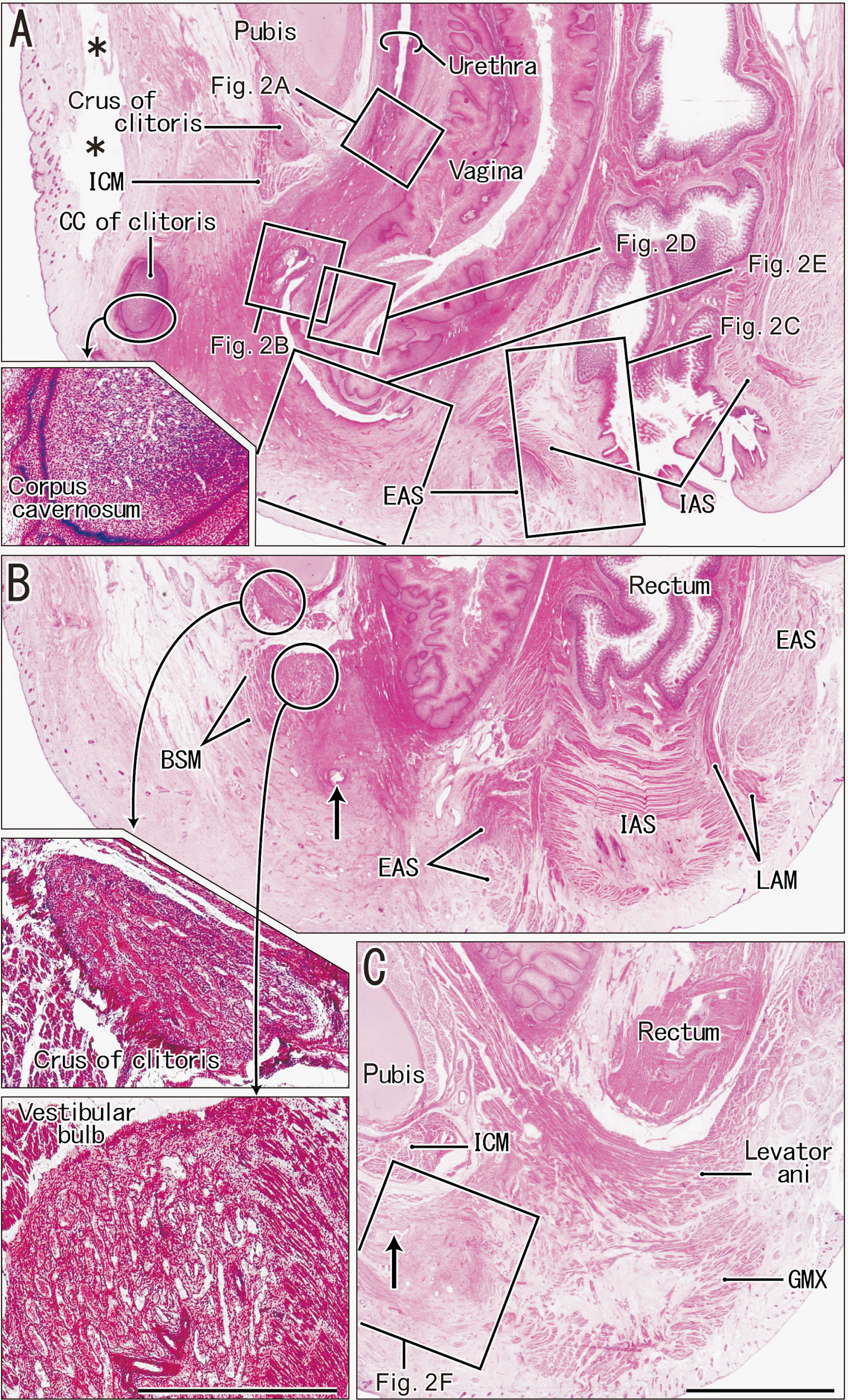
Fig. 2
Higher magnification of major structures at and around the vaginal atresia (five squares from Fig. 1). (A) shows the urethral rhabdosphincter (external sphincter). (B) shows the external orifice of the urethra with hypertrophied squamous epithelium. (C) shows a sandwiched area of the transient zone between the columnar and squamous epithelia (arrow heads). (D) shows hypertrophied squamous epithelium covering a short longitudinal septum of the lower vagina. (E) shows a thick subcutaneous tissue closing the vestibule that contains smooth muscle-like fibers. (F) shows an area immediately lateral to the vestibular bulb. The arrow in (F) indicates the lateral end of a recess of the vestibule. (A–D) were at the same magnification, and (E and F) were at the same lower magnification. Scale bars: (A–F) 1 mm. BSM, bulbospongiosus muscle; EAS, external anal sphincter; IAS, internal anal sphincter; ICM, ischiocavernosus muscle.

Fig. 3
Topographical anatomy of the uterus, vagina, and pelvic floor muscles (contralateral side of Fig. 1). (A) is 1.6 mm lateral to (B). The levator ani muscle was cut tangentially (surrounded by the dotted line in A). The uterine cervix was normal (insert, bottom right of A). A candidate of the Bartholin gland is present in the anterolateral side of the vestibule (insert, bottom right of B). (A and B) were at the same magnification and the inserts were at the same magnification. Scale bar: (A) 5 mm; (A insert) 1 mm. EAS, external anal sphincter; IAS, internal anal sphincter; GMX, gluteus maximus muscle.
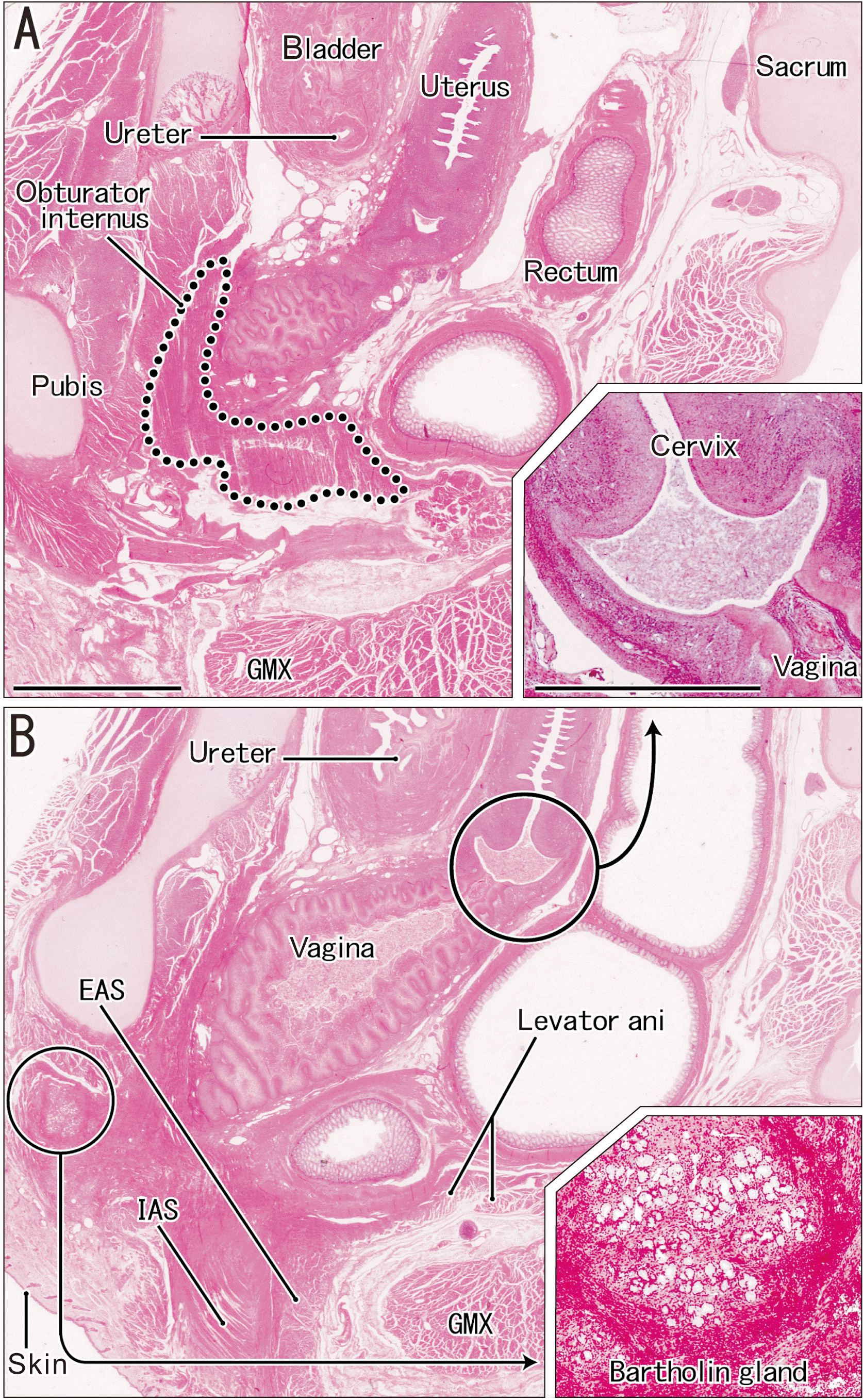
Fig. 4
Normal genital morphology at the final phase of vaginal descent in a fetus with CRL of 61 mm (sagittal sections near the midsagittal plane). (A) is the most lateral plane and (D) is the most medial plane. (E–G) are higher magnification views of the squares in (A–C), respectively. (B and C) show that the future vestibule of the vagina has a deep, gulf-like appearance. (F and G) show that the vaginal lumen is closed, and has a thick line-like appearance before recanalization. (F and G) (arrows) show the distal end of the vagina merges with the urethra near the external urethral orifice, and the vaginal descent was not yet complete. (E) shows that a longitudinal septum (star) was present in the vestibule. (E and F) show that the urethral rhabdosphincter (surrounded by the dotted line) extended inferiorly or distally along the anterior aspect of the vestibule. (C and D) show a large glans of the clitoris at the distal end of the thick CC. (A–D) were at the same magnification and (E and F) were at the same magnification. Scale bars: (A–G) 1 mm. CRL, crown-rump length; CC, corpus cavernosum.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download