This article has been
cited by other articles in ScienceCentral.
Abstract
Anatomy studies require cadavers to study the human body. Generally in Indonesia, the dead human body will be buried. This causes problems because the decomposition process of a cadaver that is preserved with formalin will be delayed and it causes environmental pollution. The toxicity of formalin can be reduced by neutralizing the formalin. This study aimed to compare the decrease of mice mass that were preserved with formalin then neutralized with sodium bicarbonate and those that were not neutralized. This study used 18 mice (Mus musculus) which were divided into 3 groups. They were the control group (not given preservative), group preserved with 4% formalin, and group preserved with 4% formalin then neutralized with sodium bicarbonate. All groups of mice were buried for 6 weeks. The changes in mass were assessed with an analysis of the percentage loss in mass. Based on the results of this study, the formalin group had a greater percentage of total mass reduction than the neutralize group. The formalin group had a higher decomposition rate than the neutralizing sodium bicarbonate group. The effectiveness of reducing the concentration of formalin is similar with neutralize group. Therefore, it can be concluded that 4% formalin is recommended for use to accelerate the occurrence of decay and decrease in mass.
Go to :

Keywords: Fixative, Mice, Formaldehyde, Sodium bicarbonate
Introduction
In medical education, learning anatomy is identical to use a cadaver to understand the human body as a whole [
1]. At the Faculty of Medicine, University of Indonesia, cadavers are an excellent learning tool for giving students a real picture of the human body. An important prerequisite in using cadavers for educational purposes is the preservation to keep cadavers safe from harm and destruction [
1]. The embalming technique that is generally used for the preservation of cadavers is using formaldehyde [
2]. The Department of Anatomy FMUI has developed 4% formaldehyde that can accelerate the decomposition process when the body is buried [
3]. Another previous studies from Wijaya et al. [
4], also developed ethanol-glycerin as an alternative fixative of formaldehyde.
The widespread use of formaldehyde for tissue fixation and embalming has had side effects on humans and the environment. Formaldehyde can be toxic, allergic, and carcinogenic. Formaldehyde causes the decomposition process of the cadaver to take longer [
1]. In this process, formaldehyde can undergo oxidation and form formic acid which can lead to the phenomenon of acid rain. Formaldehyde also kills microorganisms so that bacteria and fungi cannot degrade tissues. Nevertheless, formalin is still used because of its low cost and great effectiveness for the preservation of corpses [
5-
7]. The negative impact of formalin appears when the cadaver will be buried according to Indonesian law. This poses a problem because formalin in cadavers can pollute the environment.
The toxicity of formaldehyde can be reduced by adding salt to neutralize formaldehyde [
6] Formaldehyde (CH
2O) can be oxidized by oxygen in the atmosphere and form formic acid (CH
2O
2) [
8]. Sodium bicarbonate (NaHCO
3), also known as baking soda, is a salt commonly used to neutralize carboxylic acids [
9]. This compound has low toxicity on humans and the environment. Sodium bicarbonate is usually used to neutralize unwanted acid solutions in the laboratory chemical [
10]. Formic acid can be neutralized by sodium bicarbonate to form sodium formate which is safe for the environment [
11,
12].
In theory, sodium bicarbonate can neutralize formaldehyde in solution and produce sodium formate. Sodium bicarbonate is also readily available for this neutralization process. Through this neutralization, it is expected that cadaver to be buried can undergo a complete decomposition process. However, there has been no research associated with the neutralization of formalin with sodium bicarbonate in cadaver. Therefore, this research was conducted to compare the decrease rate in mass of mice that were preserved with 4% formaldehyde and those were neutralized with sodium bicarbonate. Thus, it is expected that the optimum approach for decreasing formalin toxicity will be discovered.
Go to :

Materials and Methods
Ethics approval
This research was approved by the Faculty of Medicine, University of Indonesia (FMUI) Ethics Committee with the letter number KET-798/UN2.F1/ETIK/PPM.00.02/2020.
Place and time of research
The research was conducted from August to December 2020 at the Department of Medical Anatomy, FMUI.
Population and research subjects
The population of this research is all preserved mice and the reachable population is all 18
Mus musculus preserved with formaldehyde in the Department of Anatomy FMUI. Because of its similarities in organ systems,
Mus musculus was chosen as the human cadaver model. The inclusion criterion was adult female mice aged 6–8 weeks with a body mass of 20–30 g. Females have a higher fat distribution than males [
13,
14]. The exclusion criterion was adult female
Mus musculus which have abnormalities, such as gait abnormalities, tumors in body organs, and pale, red, or bluish color in the mucous membrane around the eyes [
15].
Sample size estimation
In this study, researchers used the total mass of
Mus musculus as the sample and there were three groups to be studied. To determine the sample size, researchers used the resource equation. This equation was used because the standard deviation of the study could not be estimated and was not yet available in the literature [
16]. The following is the calculation of the minimum numbers of samples with the resource equation.
Note. DF, degree of freedom, the amount is between 10–20; k, number of groups; n, number of subjects in each group.
The following is an estimate of the minimum and maximum sample sizes required.
Minimum sample size for each group=10/3 +1=4.3 rounded up to 5.
Maximum sample size for each group=20/3+1=7.7 rounded down to 7.
Based on the calculations above and considering the principles of the replacement, the reduction, the refinement, the number of samples required for each group was 6. In this study, three groups were conducted. The first group as the control was mice that were not given preservation. The second group was mice given a formaldehyde fixative without a neutralizer. The third group was mice that were given formaldehyde and neutralized with sodium bicarbonate. Therefore, the total sample size required was 18 mice.
Fixation of mice
At the beginning of the research, the concentration of sodium bicarbonate was determined by making a solution of sodium bicarbonate with concentrations of 30%, 40%, 50%, and 60%. Then, each solution was mixed with 4% formalin. Formaldehyde levels were measured with a formaldehyde meter kit (Quantofix Formaldehyde). The concentration of sodium bicarbonate was used when the formalin level is close to 0%. In this case, the 50% sodium bicarbonate concentration could reduce the formalin level. The stages of the research were carried out according to the group of mice. The three groups of mice were anesthetized by injection of Xylazine 10 mg/kg and Ketamine 100 mg/kg. Then mice were given an injection of 0.9% NaCl for drainage of the mice’s circulation. A total of 6 mice were not given formalin fixative solution, dissected, and buried for 6 weeks. Another 6 mice were injected with formalin and soaked in 4% formalin solution for 1 week. After 1 week, the mice were drained from the formalin bath. Then, the mice were dissected and buried for 6 weeks. The other 6 mice were a group that was preserved with formalin and neutralized. After preservation, the level of formalin was measured using a formaldehyde meter kit. Then this group was immersed in a 50% sodium bicarbonate neutralizing solution. At the first week, the level of formalin is still high. Thereafter measurement of formalin levels was carried out again in the next week and the formalin levels were close to 0%. After that, the mice were dissected and buried for 6 weeks. The soil used for burial contents fertile soil, a combination nitrogen, urea, and organic phosphates, microorganisms, calcium carbonate, and cocopeat (with ratio: 50%:20%:10%:10%:10%).
Mass measurement observation
Every week, observations were made by measuring the decrease in the total mass of mice and observe the decomposition that occurs. To overcome the less sensitive measuring instrument, the mass measurement was carried out 3 times and carried out by 3 observers.
Observation of the body of mice was carried out by assessing the stages of decomposition according to fresh, bloated, active decay, advance decay, and dry/remains. The fresh stage occurs immediately after death is experienced by an organism and the decomposition activity is still minimal. The bloated stage is characterized by the body starting to swell due to buildup of gas produced by microorganisms. In active decay stage, the rate of tissue degradation is increasing and the tissue is starting to deflate. In advance decay stage, the degree of degradation is complete, body looks dry, and dark in color. The dry/remains stage is characterized the organs are gone and it is odorless [
3,
15].
Statistical analysis
In this study, the data analyzed was the decrease in body mass of mice. Researchers performed data processing by using the percentage of mass decrease to describe the mass changes that occur. The collected numerical data were tested for normality using the Shapiro Wilk method. The data were normally distributed (P>0.05), so the analysis used was the One-Way ANOVA test. The data stated that there was a significant difference if it had a P-value<0.05. Furthermore, analysis was continued with post hoc tests, such as Games-Howell and Bonferroni test.
Go to :

Results
Sodium bicarbonate concentration
At first, researchers conducted experiments to determine the appropriate concentration of sodium bicarbonate. Formaldehyde level was measured with a formaldehyde meter kit. In the study, it was found that the neutralizing concentration of 50% sodium bicarbonate could reduce formalin levels close to 0%. There could be an optimum time for sodium bicarbonate to neutralize formaldehyde in the tissues.
Observation of the total mass of mice
In this study, the sample of study was female mice with Mus musculus species aged 6–8 weeks with a body weight of 20–30 g. Observations were made on three groups of mice, namely the control group, formalin and the group that was given a neutralizer. The formalin group showed the tissues looked paler, the mice’s bodies felt stiffer, and the mice’s bodies had sunk to the bottom of the container. The body of the mice was well preserved with formalin in 4–7 days. The neutralized group showed the tissues looked paler, but the bodies were not too stiff. The body of mice was neutralized within 2 weeks.
In accordance with the data analysis that has been carried out, the results show the distribution of the data is normal. Therefore, the total mass of each group of mice are shown with the mean±standard deviation. The following is the average total mass of mice shown in
Table 1.
Table 1
Average total mass of mice per week
|
Week |
Control group (g) |
4% formalin group (g) |
Formalin and sodium bicarbonate group (g) |
|
0 |
22.833±0.753 |
36.333±3.141 |
32.307±2.295 |
|
1 |
11.167±1.835 |
22.667±1.643 |
22.821±1.340 |
|
2 |
4.086±2.168 |
19.822±1.670 |
20.201±1.298 |
|
3 |
2.631±1.548 |
15.778±0.202 |
15.435±1.793 |
|
4 |
2.521±1.483 |
10.174±1.037 |
9.985±1.310 |
|
5 |
2.328±1.463 |
7.665±0.910 |
8.190±0.507 |
|
6 |
2.051±1.311 |
4.996±1.236 |
5.268±0.613 |

Based on observations of the total mass shown in
Fig. 1, the formalin group and sodium bicarbonate group experienced a gradual decrease in total mass. The following is the percentage decrease in mass from the three groups.
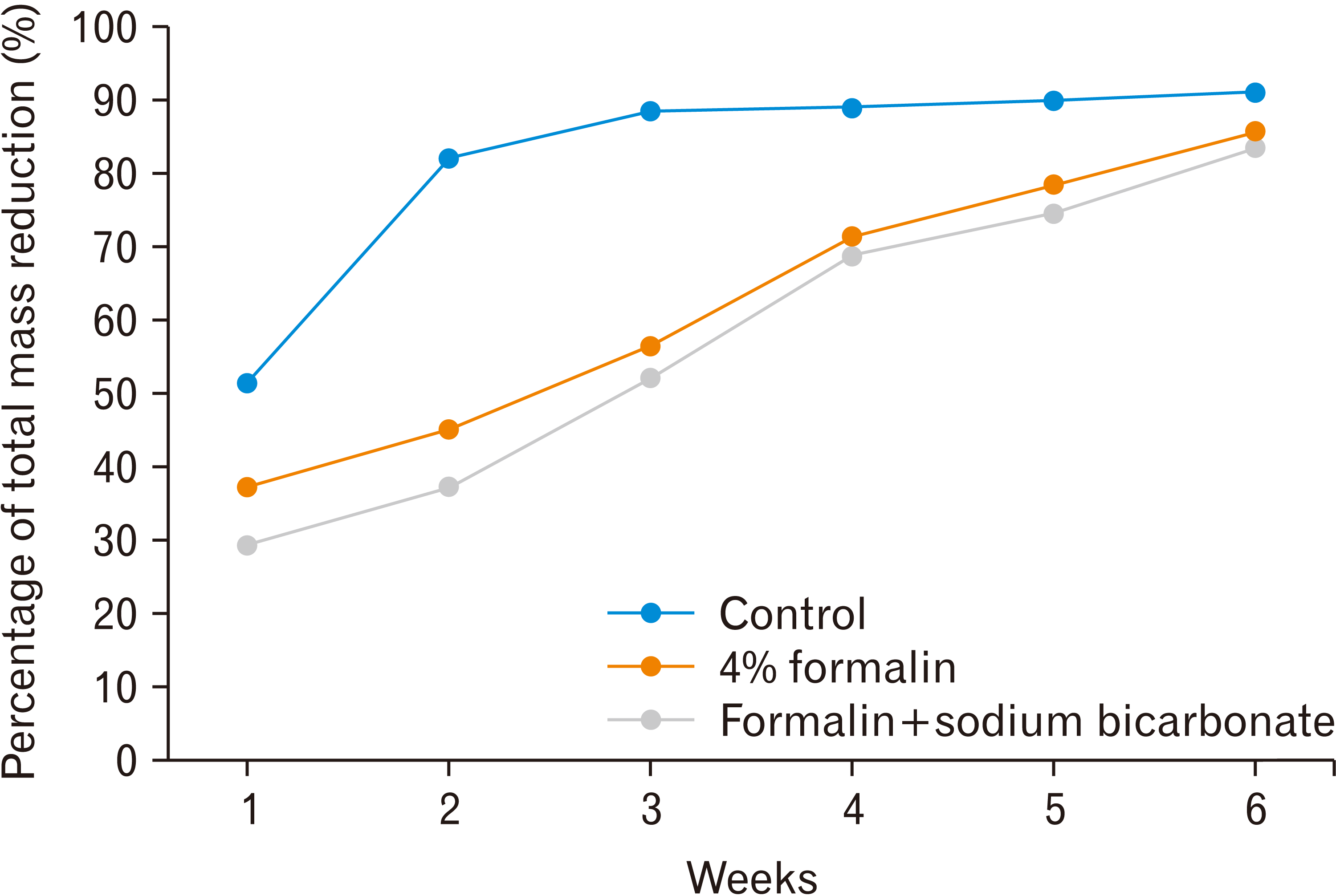 | Fig. 1Percentage of total mass reduction each week. 
|
Based on the percentage decrease in total mass shown in
Table 2, the control group experienced the greatest decrease compared to the other groups. In addition, the formalin group had a greater percentage of total mass loss than the neutralizing sodium bicarbonate group. The results of the ANOVA test showed that there were at least two groups that had significantly different percentages of mass loss. To find out which group had the difference, it was followed by the post-hoc test. Overall, there was a significant difference between the control group and the sodium bicarbonate neutralizing group. However, there was no significant difference in the percentage of total mass reduction between the formalin group and the sodium bicarbonate neutralizing group.
Table 2
Percentage decrease in the total mass of mice
|
Week |
Control group |
4% formalin group |
Formalin and sodium bicarbonate group |
P-value ANOVA test |
|
1 |
51.125±7.652 |
37.488±2.915 |
29.275±2.607 |
<0.001a
|
|
2 |
82.079±9.473 |
45.136±6.105 |
37.337±4.141 |
<0.001 |
|
3 |
88.517±6.623 |
56.292±4.568 |
52.252±4.015 |
<0.001 |
|
4 |
89.015±6.286 |
71.753±4.298 |
68.890±5.314 |
<0.001 |
|
5 |
89.866±6.219 |
78.617±4.234 |
74.630±0.644 |
<0.001 |
|
6 |
91.065±5.614 |
85.986±4.366 |
83.673±1.800 |
0.025 |

Observation of the mice
Observations on the level of decomposition of mice were carried out as a whole. Observation of the decomposition rate of mice was carried out for 6 weeks by assessing the stages of decomposition of each group of mice. The following is observation from the stages of decomposition of mice for 6 weeks.
Observation on mice was carried out by looking at the decomposition stages converted to numeric, namely 1=fresh, 2=bloated, 3=active decay, 4=advanced decay, and 5=dry, remains/skeletal. Based on the observation, the data was analyzed in graphical form to make it easier to understand. From the graph, it can be seen that the level of decomposition of each group increases every week. The following is a graph of the stages of decomposition of the total mass of mice each week.
From the graph shown in
Fig. 2, it can be seen that there are differences between the stages of decomposition per week in the three groups. The control group had the highest decomposition rate compared to the other groups. The formalin group and the sodium bicarbonate group continued to experience a gradual increase in the decomposition stage every week. The difference in decomposition stages between the formalin group and the sodium bicarbonate group could be seen in the second week. The formalin group still had a higher decomposition rate than the neutralizing sodium bicarbonate group.
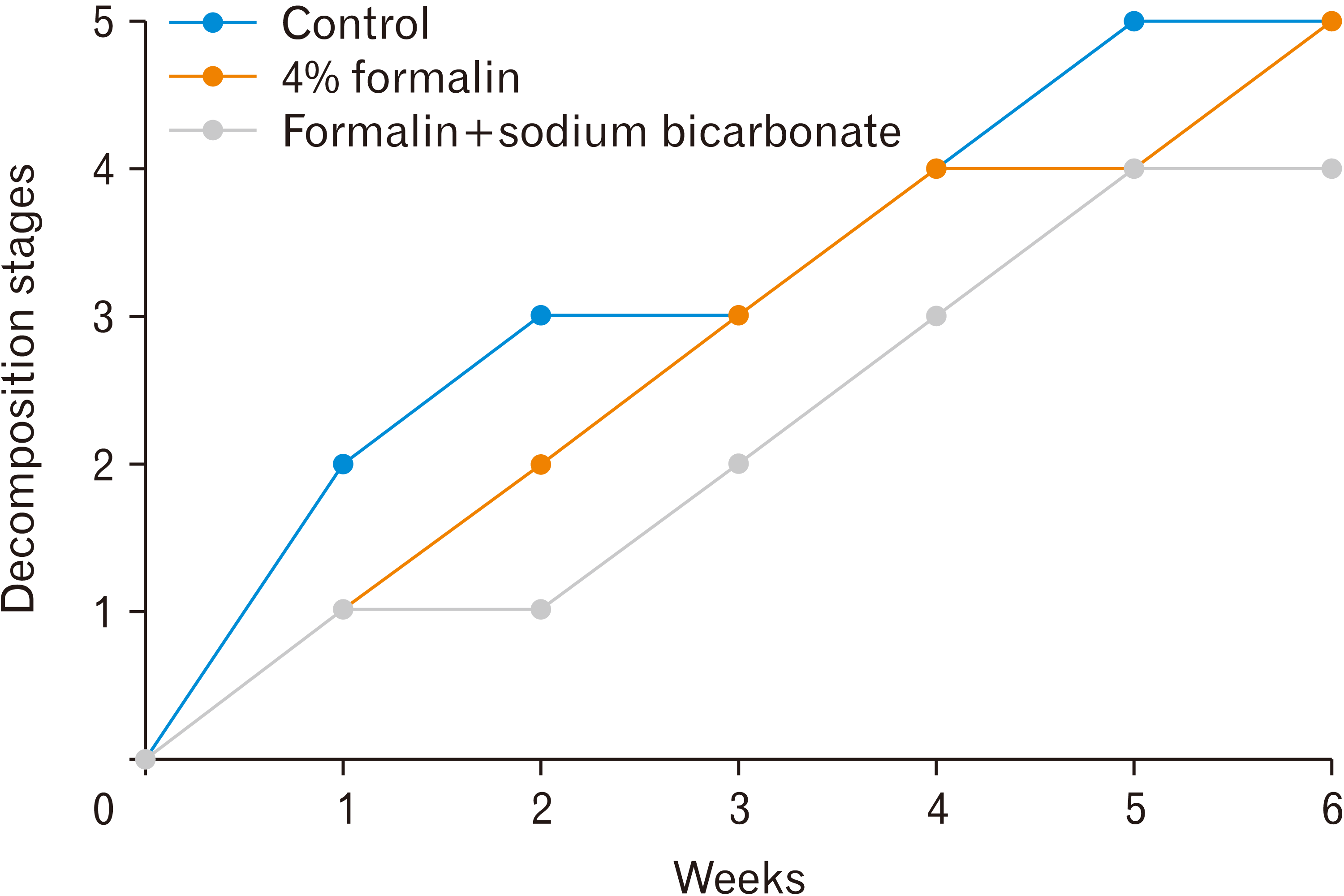 | Fig. 2Stages of decomposition of the total mice each week. 
|
Go to :

Discussion
Decomposition is carried out by detritivores and decomposers which will degrade the tissue. One of the stages of decomposition process, namely active decay, is characterized by an increase in the activity of microorganisms that degrade tissue and cause a decrease in mass. Therefore, the decrease in mass can be a criterion for assessing the level of decomposition that occurs. During decomposition, microorganisms multiply and play a role in the recycling of carcasses through enzymatic tissue degradation. This tissue degradation causes a decrease in mass [
17,
18].
Formaldehyde is a fixative commonly used for cadaver preservation to prevent tissue decomposition. Formaldehyde will form cross-link bonds with proteins, fats, and nucleic acids. This cross-link causes the tissue decomposition process to be delayed. However, formaldehyde creates problems when the cadaver is buried according to Indonesian law. This has negative impacts on the environment, such as contaminating groundwater and affecting plant growth [
19,
20].
The use of sodium bicarbonate or known as baking soda is very wide. This salt is also easy to find at a fairly affordable price. However, based on the study, the 4% formalin has greater decrease in mass of mice than those are neutralized with sodium bicarbonate. Researchers suspect this is due to the less optimal neutralization process and the content of bicarbonate compounds in the sodium bicarbonate salt.
In laboratories, sodium bicarbonate is commonly used to neutralize unwanted acids because it has high reactivity to acid pollutants. Sodium bicarbonate reacts with acid pollutants by acid-base neutralization reaction. However, this neutralization process requires an optimal temperature. Sodium bicarbonate has a characteristic thermal decomposition. At higher temperatures, sodium bicarbonate will decompose into sodium carbonate, carbon dioxide, and water. This sodium carbonate reacts with the acid in a neutralization reaction to form salt. At lower temperatures, it is likely that sodium bicarbonate only dissolves in formic acid. With an increase in temperature, this neutralization reaction can be optimized. The inadequate temperature causes sodium bicarbonate to be unable to decompose so that the neutralization reaction is not optimal [
21].
Simultaneously, bicarbonate that is exposed to moisture will increase the pH of the environment (alkaline environment). This will create a hostile environment for microorganisms. Soil containing sodium bicarbonate is very soluble, and it increases the soil pH above 8. At the time of this research, it rained frequently. This can increase the level of soil moisture or water that fills the soil pores. Therefore, the increased humidity level and the increased amount of water in the soil can cause the soil’s acidity level to a low degree or the soil to be alkaline [
22,
23].
Microorganisms play an important role in soil processes, such as substance replacement, biogeochemical cycles, plant growth, and soil ecosystems. Microbial growth will decrease when the environmental pH changes from the optimum pH. In the environment, changes in environmental pH to 1 unit low or high can reduce the metabolic activity of the microbial community by up to 50%. pH can also influence microbial metabolism through redox reactions. Microbial respiration catalyzes redox reactions to synthesize ATP. Environmental pH helps in controlling respiration and microbial growth [
24].
Increasing soil pH affects microbial activity that plays a role in tissue degradation. The presence of the organism indicates that the cadaver has entered active decay. At this stage, there is a large decrease in mass due to the increased activity of the organism. As a result of alkaline soil pH can affect the activity and the growth of microorganisms [
18].
The percentage decrease in total mass and the decomposition rate was found to be higher in formalin 4%. This shows that a decrease in formalin concentration is effective compared to the addition of a neutralizing agent. Formalin changes the structure of the tissue to become denser and prevent the decomposition process. This happens because formalin reacts by creating cross-link in protein [
3,
19]. The decrease concentration of formalin has lower preservation ability and reduce stiffness in tissues. The protocol of formalin concentration reduction can be applied compared to the addition of other substances as neutralizing agents.
The benefit of this research is this research can be used as a reference for further research and consideration for researching more effective formalin neutralization. This research also has limitations in its implementation. The limitation did not control environmental factors, such as temperature and acidity. This study also did not measure the level of acidity, either before or after neutralization.
In conclusion, the reduction in total mass of preserved mice with 4% formalin is higher than those were given a solution of sodium bicarbonate as a formalin neutralizer. Researchers recommend in using decrease in formalin concentration rather than addition of other solutions. However, further toxicity studies need to be conduct to investigate the detrimental effects of formalin before is able to be done in medical education human cadaver.
Go to :

Acknowledgements
We are grateful and would like to give our best gratitude to Universitas Indonesia Research Grant, International Indexed Publication (PUTI) program 2020. This article was presented in the 5th International Conference and Exhibition on Indonesian Medical Education and Research Institute (5th ICE on IMERI), Faculty of Medicine, Universitas Indonesia. And we sincerely appreciate the 5th ICE on the IMERI committee, who has supported the peer-review and manuscript preparation before submitting it to the journal.
Go to :

Notes
Go to :

References
2. Hayashi S, Naito M, Kawata S, Qu N, Hatayama N, Hirai S, Itoh M. 2016; History and future of human cadaver preservation for surgical training: from formalin to saturated salt solution method. Anat Sci Int. 91:1–7. DOI:
10.1007/s12565-015-0299-5. PMID:
26670696.
3. Yogawisesa SC. 2018. Effect of 10% and 4% formalin solution in decomposition process of mice hindlimb. Universitas Indonesia;Jakarta: DOI:
10.1007/s12565-015-0299-5.
4. Wijaya AN, Margiana R, Kusumaningtyas S, Furqonita D. 2021; Comparison of decomposition rate of hind limbs of preserved mice with ethanol-glycerin and formaldehyde of advanced fixative solution. Anat Cell Biol. 54:225–31. DOI:
10.5115/acb.20.314. PMID:
33767018. PMCID:
PMC8225480.
5. Elshaer NSM, Mahmoud MAE. 2017; Toxic effects of formalin-treated cadaver on medical students, staff members, and workers in the Alexandria Faculty of Medicine. Alex J Med. 53:337–43. DOI:
10.1016/j.ajme.2016.11.006.
6. Musiał A, Gryglewski RW, Kielczewski S, Loukas M, Wajda J. 2016; Formalin use in anatomical and histological science in the 19th and 20th centuries. Folia Med Cracov. 56:31–40. PMID:
28275269.
7. Liteplo R, Beauchamp R, Meek ME, Chénier R. 2002. Formaldehyde: concise international chemical assessment document 40. WHO;Geneva: p. 34–5.
8. Andrushkevich TV, Popova GY, Danilevich EV, Zolotarskii IA, Nakrokhin VB, Nikoro TA, Stompel SI, Parmon VN. 2014; A new gas-phase method for formic acid production: tests on a pilot plant. Catal Ind. 6:17–24. DOI:
10.1134/S2070050414010024.
11. Hu L, Adeyiga AA. 1997; Extraction of formic acid from sodium formate. Ind Eng Chem Res. 36:2375–9. DOI:
10.1021/ie9605443.
17. Surabian D. 2012. Preservation of buried human remains in soil. Natural Resources Conservation Service;Tolland: p. 1–14. DOI:
10.1016/j.lfs.2015.10.025.
18. Hau T, Hamzah N, Lian H, Hamzah S. 2014; Decomposition process and post mortem changes: review. Sains Malays. 43:1873–82. DOI:
10.17576/jsm-2014-4312-08.
19. Thavarajah R, Mudimbaimannar VK, Elizabeth J, Rao UK, Ranganathan K. 2012; Chemical and physical basics of routine formaldehyde fixation. J Oral Maxillofac Pathol. 16:400–5. DOI:
10.4103/0973-029X.102496. PMID:
23248474. PMCID:
PMC3519217.
21. Pozzo AD, Moricone R, Tugnoli A, Cozzani V. 2019; Experimental investigation of the reactivity of sodium bicarbonate toward hydrogen chloride and sulfur dioxide at low temperatures. Ind Eng Chem Res. 58:6316–24. DOI:
10.1021/acs.iecr.9b00610.
22. Ajileye AB, Esan EO, Adeyemi O. 2018; Human embalming techniques: a review. Am J Biomed Sci. 10:82–95. DOI:
10.5099/aj180200082.
23. Rengasamy P. 2016; Soil chemistry factors confounding crop salinity tolerance: a review. Agronomy. 6:53. DOI:
10.3390/agronomy6040053.
24. Jin Q, Kirk MF. 2018; pH as a primary control in environmental microbiology: 1. thermodynamic perspective. Front Environ Sci. 6:1–13. DOI:
10.3389/fenvs.2018.00021.
Go to :





 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download