Abstract
This study aimed to investigate the general mandibular symphysis (MS) shape variation among Class III skeletal base, using geometric morphometric analysis. Pre-treatment lateral cephalometric radiographs of 254 patients aged 11–40 years old, with Class III skeletal base (ANB <1o) and lower incisor angle (<99o) were included. Nine-landmarks with x and y coordinates were identified on MS using TPSDig2 software, then exported into Morpho J for shape and statistical analysis. Principal component analysis showed that three main shape dimensions with a total variance of 74.6% represented the majority variation of samples. Procrustes Anova showed the shape of MS in Class III skeletal base to be mainly influenced by gonial angle, incisor inclination and sex (P<0.0001). Canonical variate analysis showed that high gonial angle groups had significantly narrower and elongated MS whereas low gonial angle groups had wider, bulbous and rounded MS (P<0.0001). The ratio of alveolar part to basal part was 1:5 in low gonial angle and 2:3 in high gonial angle. Males had significantly taller MS with narrower B point area compared to females (P<0.0001). Retroclined incisors exhibited taller and retroclined alveolar parts (P<0.0001). The shape of MS in Class III skeletal base varied at the alveolar part, basal part or both and it is influenced by gonial angle, incisor inclination and sex. Hence, understanding the shape variation of MS is important to aid orthodontic treatment planning.
The management of non-growing patients with skeletal Class III involves camouflaging or skeletal correction via orthognathic surgery. During orthodontic camouflage, retroclination of the lower incisors are needed to achieve a class I incisor relationship. Conversely, in patients with severe Class III skeletal discrepancies, decompensation of the lower incisors prior to orthognathic surgery is required by uprighting or proclining the lower incisors.
Unfortunately, orthodontic tooth movement in Class III malocclusion cases is always challenging. The anteroposterior thickness of the alveolar bone in the lower incisor region has been reported to be thin in mandibular prognathism and may limit the range of incisor movement [1]. Class III malocclusion patients with long lower face height are frequently present with narrow alveolus, linguoversion of mandibular symphysis (MS) and thin labial bone. Therefore, pronounced tipping movements of the lower incisors can cause labial gingival recession and alveolar bone loss [2]. Presence of thin gingival biotype and dental crowding could further complicate matters.
The understanding of MS morphology is important during orthodontic treatment planning as it provides essential information in determining the limits of orthodontic proclination or retroclination of the lower incisors. It is also an important landmark for surgical planning such as genioplasty, block bone grafting [3], and dental implant insertion. If the lower incisor roots are moved against the cortical plate of the alveolus, severe root resorption and bony dehiscence may occur. Limiting lower incisor movement within the MS during orthodontic treatment is thought to be paramount for achieving better results, stability, and periodontal health [4].
Variation in MS morphology has been associated with individual variation [5], vertical facial proportions [5-10], sagittal skeletal relationship [11], incisor inclination [1, 12, 13], muscular and functional activities [4, 14, 15], and sexual dimorphism [3, 16]. Studies on facial type and morphology of MS have mentioned that those with short face or forward growth of mandible has wider MS while those with long face or backward growth of mandible exhibits elongated and thin MS [8-10, 17]. Moshfeghi et al. [18] further emphasized that mandibular morphology is associated with the direction of mandibular growth. Other studies also suggest that MS in growing children may be predictive for lower face height in adulthood [19]. Nevertheless, it is interesting to know which factors most influence mandibular symphysis.
Majority of studies employ conventional linear and angular measurements as an assessment tool. As MS is curved and round, linear measurements will have limitations in defining and describing the actual shape of MS. Studies also show that conventional cephalometric study based on angular and linear measurements were insufficient to analyse shape changes of complex anatomical forms such as craniofacial complex [20, 21]. Besides, size is usually the confounder in statistical analysis [22]. Craniofacial shape can be affected by various factors such as sexual dimorphism, patient’s weight and built, and genetics. Moreover, visual interpretation of results may be limited, especially when describing shapes.
Geometric morphometric analysis (GMA), in contrast, has the advantages to analyse and compare the overall shape and variation of MS without being affected by its size. This method is able to study the changes as well as the variability of MS shape, with its ability to measure in two-dimensional (2D) and three dimensional (3D) objects. GMA is a tool that incorporates mathematics and biology, commonly used in morphological studies investigating shape variations of objects. It uses Cartesian landmark coordinates that can capture morphologically distinct shape variables, providing a graphical representation of statistical interpretation [23]. Size of the structure would not be a confounder in statistical analysis as GMA analysis is based on landmarks after separating shape coordinates from size, position, and orientation of the landmark configurations.
To best of our knowledge, no other studies have used GMA to study the shape of MS. Therefore, the aim of this study is to investigate the general MS shape variation among Class III skeletal base using geometric morphometric analysis. In addition, we aim to determine which factors most influence the shape variation of MS. The comparison of different vertical facial proportions using GMA would give a clear graphical interpretation of MS shape, that can add value to current literature.
This was a cross-sectional study where lateral cephalometric radiograph (LCR) was taken on patients who seek orthodontic treatment in the Faculty of Dentistry, Universiti Kebangsaan Malaysia (UKM), from 2010–2019. Ethical approval was obtained from the Research and Ethics Committee of UKM (UKM PPI/111/8/JEP-2018-509).
The inclusion criteria for the study were Class III skeletal with ANB <1°, lower incisor angle <99°, aged 11–40 years old, with no significant medical history nor taking bisphosphonates and no history of previous orthodontic treatment. Subjects with missing lower incisors, history of periodontitis, dental anomalies, syndromic and craniofacial deformities as well as poor image quality of the LCR were excluded.
For geometric morphometric studies, the sample size calculation cannot be determined by straightforward application of mathematical formula [24] as there is no clear guideline [25]. A study suggested a minimum sample size calculation based on the number of coordinates and landmarks [26]. Therefore, in this study, 9 landmarks with 2D coordinates (x, y) will yield a minimum sample of 18 (9×2=18) per group to allow for inferential statistics.
A total of 254 LCR were obtained using Planmeca Romexis software (Planmeca ProMax 3D Classic; Planmeca Romexis, Helsinki, Finland) following these exposure parameters: 60–90 kV, 1–14 mA, exposure time between 9–37 seconds and a source-midsagittal plane distance of 1.5 m. The LCR were digitally traced using VistaDent OC (2D) (GAC International, Inc., Bohemia, NY, USA). The subjects were classified based on gonial angle: high (>130°), average (120°–130°) and low (<120°) [12]. The gonial angle were measured from the inclination of the posterior border of ramus and the inferior border of the horizontal body of the mandible [27].
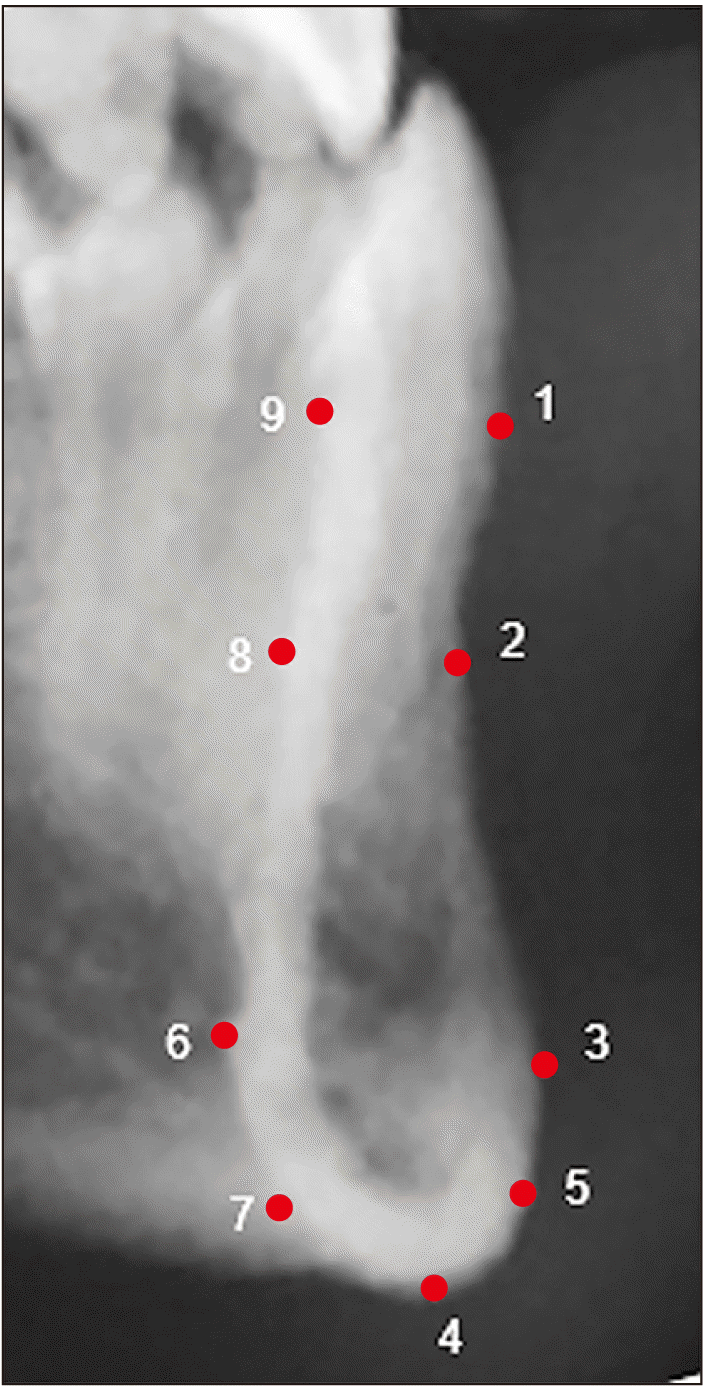
Nine 2D fixed landmarks were chosen based on the standard landmarks used for lateral cephalometric tracing, to comprehensively capture the shape of MS. Digital LCR were converted into a TPS file and imported into TPSDig2 software [28]. Most landmarks on MS were type III landmarks, which are defined as points along a curve or surface, in relation to some other more distant structure [29]. Hence, the Frankfort horizontal plane was used to define the precise location of all landmarks (Table 1, Fig. 1).
The x and y coordinates of each landmark generated from TPSDig2 version 2.30 [28] were exported into Morpho J version 1.06d [30] for shape analysis. Generalised procrustes analysis (GPA) was performed where all samples were superimposed, translated, scaled and rotated to remove irrelevant information such as size, orientation and position for shape comparison. Scaling process ensured all subjects have the same centroid size of 1.0. Translation and rotation process was done according to the criterion of best fit (minimal sum of squared distances between corresponding landmarks) to achieve a standard position and orientation. The differences in landmark position between shape coordinates after GPA indicating shape difference are also known as Procrustes distance.
Principal component analysis (PCA) was carried out to extract the most significant components and further categorized the variation among Class III subjects. PCA transforms the shape variables mathematically to create a new set of independent variables, known as principal components (PC), that carries all the original shape information. Each PC describes its distinctive shape variation of the study subjects [31]. The first PC (PC1) accounts for the most variance of the MS while PC2 has lesser variance and so on. Further analysis will be based on the first few PCs that accounts for most of the total variance. Procrustes ANOVA assessed the effect of age groups on MS variation. Canonical variate analysis (CVA) assessed the shape differences among gonial groups. It compared the group pairwise differences and produced a scatter plot which enables visualization of the shape variation between the groups. CVA produced a set of new variables, called canonical variate (CV), which accounts for the maximum amount of among-group variance relative to within-group variance, by assuming that the groups all share the same covariance matrix. Mahalanobis distances were used to measure the mean shape differences between groups. P-value was calculated based on 10,000 permutations. Alpha level for all statistical analyses was set at 0.05.
Intra-examiner and inter-examiner reliability were carried out on 26 randomly selected LCR at 2 weeks apart to ensure LCR tracing and landmark placement accuracy. For LCR tracing, intraclass correlation coefficients test (ICC) showed above 0.95 and above 0.89, both intra and inter-reliability tests, respectively. ICCs for landmark placement also showed high degree of reliability with 0.999 for x coordinates and 0.987 for y coordinates.
A total of 254 LCR were included in this study. The mean age of subjects was 21.78±5.95 years old. The sample consisted of 94 (37.0%) males and 160 (63.0%) females with mean ANB values of –2.44±2.31. More than half of subjects 132 (52.0%) had retroclined lower incisors whereas 122 (48.0%) had upright lower incisors. Majority (45.7%) had average gonial angle at 124.96°±2.97°, 29.5% had high gonial angle at 133.99°±3.29° and 24.8% had low gonial angle at 114.59°±3.62°.
GPA produced the scatter of landmarks, showing the superimposition of x and y coordinates of each landmark on MS (Fig. 2). The blue dot is the acquired mean position and orientation of MS. The blue circle encompassing landmarks (numbered in red) indicate landmark variation of the 254 samples. The shape variation of the MS was most pronounced at the lingual surface of the basal part (landmark 6), as the landmarks were clustered in an elongated oval shape. Vertical variation was also observed at the alveolar part of MS (landmark 1, 2, and 9). In contrast, landmark 4 (menton), 5 (gnathion) and 7 (the most posterior inferior point of bony chin) showed the least variation as the landmarks were clustered in a near-circular shape.
Principal component analysis displayed a multivariate analysis and major features of shape variation in a data set. Thus, PCA produced 14 dimensions of MS shape variation, represented by PC1 to PC14. PC1 to PC3 contributed 36.0%, 20.4%, and 18.3% of the total variance, respectively. This represented the majority of the variation (74.6%) within the whole data. Wireframe drawings of the MS connects the landmarks with straight lines. The light blue outline is the mean shape of the sample while the dark blue outline representing the shape changes associated with PC1, PC2, and PC3 (Fig. 3). The positive change of PC1 showed narrower and more elongated alveolar and basal part of MS whereas the negative change of PC1 showed wider and shorter MS. The positive change of PC2 consisted of an inferiorly positioned B-point, taller and retroclined alveolar part and narrower, elongated, and an oval-shaped basal part of the MS. Meanwhile, the negative change of PC2 comprised of superiorly positioned B-point, shorter and upright of alveolar part and rounded basal part of the MS. Lastly, the positive change of PC3 comprised of a more superiorly positioned B-point (shorter) and a narrower alveolar symphysis while the negative change of PC3 had a more inferiorly positioned B-point (taller) and wider alveolar symphysis.
Procrustes ANOVA assessed the factors that affect the shape variation of MS such as sex, incisor inclination, gonial angle. The gonial angle has the largest effect on the shape of MS with 9.2% of a total sum of squares, followed by age group (2.16%), incisor inclination (1.49%) and sexual dimorphism (1.29%) (Table 2). Discriminant analysis showed that male’s MS is taller and narrower at the lingual basal part and B-point area compared to female (P<0.0001). The more retrocline the lower incisor, the more elongated the alveolar part of MS (P<0.001). However, the shape of the basal part of the MS is similar.
CVA analysed the shape differences in MS between 3 gonial angle groups: high (79 samples), average (116 samples) and low (63 samples). The first canonical variate (CV1) accounted for 86.4% and the second CV (CV2) accounted for 13.6% of the amount of relative between-group variation (Fig. 4). The shape changes associated with CV1 involved the width and height of MS. The CV scores showed a gradient from wide and short MS to narrow and elongated MS along CV1. For CV2, it showed the changes in the basal symphysis width and the level of B point. At the negative end of CV2, the MS exhibits superiorly positioned B point, and more elongated basal part and flattened pogonion area. At the positive end, the MS exhibits inferiorly positioned B point, wider and more rounded basal part with prominent pogonion.
MS of high gonial angle was mainly concentrated at the positive end of CV1 (–1.0 to +4) compared to the low gonial angle which was concentrated at the negative end (–3.5 to +1.0). In average gonial angle group, MS spread from –3 to +3, along CV1. Permutation tests using Mahalanobis distance (10,000 permutations per test) showed significant shape variation between all 3 groups (P<0.0001). High gonial angle group had significantly narrower and elongated basal and alveolar part of the MS whereas the low gonial angle group had wider, bulbous and rounded basal part and short and wider alveolar part of MS. In addition, the ratio of alveolar part to basal part is 1:5 in low gonial angle and 2:3 in high gonial angle. The average gonial angle group had more inferiorly positioned B point, wider and more rounded basal part with prominent pogonion.
We aim to investigate the general shape variation of MS among Class III skeletal patterns and determine which factors may have the highest influence on the shape variation of MS. GPA superimposed all samples and removed non-shape information to allow visualization of MS shape variation. The scatter of points appeared more pronounced and elongated in the lingual basal part of MS and alveolar region with lesser variation landmarks observed at the lower border of MS. Therefore, more shape variation was observed around the lingual basal part of MS where the width of MS varies horizontally, from slim to wide. Significant shape variation of alveolar bone was observed surrounding the lower incisors, with vertical variation from short to tall. This is influenced by dentoalveolar compensation of teeth in Class III skeletal pattern.
In this study, PCA identified 14 different shape of MS. The first three PCs represented the majority variation of the sample (74.6%) and were the main features to classify most of the MS in Class III skeletal base. PC1 describes the variation in width and height of the whole MS (narrow and elongated or wide and short). PC2, on the other hand, describes the variation at the level of B-point (inferiorly or superiorly position), inclination of alveolar region (retrocline or procline) and shape changes at the basal of MS (oval or rounded). PC3 describes the variation in height and width of the alveolar part of MS (short and narrow or wide and tall). Bangar et al. [11] found that MS in Class III skeletal bases has reduced concavity on the labial surface and increased vertical dimension compared to Class I and Class II skeletal bases. Maniyar et al. [17] also found that Class III skeletal base with retroclined incisors has taller MS. This is not in agreement with our result where our study indicates that MS variation in Class III skeletal base can be observed at the alveolar part, basal part, or both, where it varies from narrow and elongated or wide and short as mentioned previously. Previous studies indicate that the shape variation of MS could be due to individual variation [5], vertical facial proportions [5-10], sagittal skeletal relationship [11], incisor inclination [1, 12, 13], muscular and functional activities [4, 14, 15], and sexual dimorphism [3, 16]. However, these studies used linear measurements to measure height, width, or inclinations of MS. Linear and angular measurements limit the visual interpretation of result, especially when describing the round and curved MS shapes.
Utilization of GMA not only allowed visualization of MS shape variation, but has also identified that gonial angle (9.2%) has the largest effect on the shape of MS, followed by incisor inclination (1.49%) and sex (1.29%). Gonial angle, also known as angle of the jaw, is an angle measured between the inclination of the posterior border of ramus and inferior border of the horizontal body of the mandible [27, 31]. It was used in this study to define the divergence of mandible and not influenced by other facial structures and landmarks. The gonial angle was also found to be associated with vertical facial proportion where it was significantly increased in hyperdivergent subjects [9].
CVA showed significant shape variation between high, average and low gonial angle groups (P<0.0001). The high gonial angle group had narrow, slim and elongated MS. The average gonial angle group had a relatively shorter alveolar part, wider and more rounded basal part with prominent pogonion compared to high gonial angle group. Low gonial angle, on the other hand, had the shortest and widest the alveolar part. The basal part was also wide, rounder, and bulbous. This finding is similar with previous studies which concludes that those with long face or backward growth of mandible (high gonial angle) exhibits elongated and thin MS [5-9]. Aki et al. [19] also confirmed our findings in that MS morphology is associated with mandibular growth rotation in anterior and posterior directions.
Additionally, different facial types have been shown to be associated with different occlusal forces, where those with long faces have lower bite force magnitude and reduced masticatory muscle size [15, 32]. Skeletal Class III open bite patients with reduced biting forces were reported to have thinner MS compared to patients with normal overbite [4]. Increased neuromuscular activity and training such as masticatory forces, can result in muscle hypertrophy, metabolic and cross-sectional area changes, and may change the fiber-type composition of the muscle towards a larger percentage of slow-type fibers [33]. Low angle group with increased muscle activity [34] have larger muscle cross-sectional area [35] as well as wider trapezoidal-shaped ramus, bigger coronoid, rectangular body, curved basal arch and wider MS.
The findings of this study also show that the ratio of alveolar to basal part was different in the three gonial angle groups. The high gonial angle group has the tallest alveolar part and vice versa for the low gonial angle group. This suggests that craniofacial structures grow differently between low and high angle facial types, where anterior lower facial height growth is greater in high angle group [36]. Additionally, the alveolar part of MS in the retroclined incisor group is longer or taller compared to those in the upright incisors’ group.
As the vertical facial proportion increases, natural dentoalveolar compensation occurs where more teeth extrude and MS elongates to meet with opposing teeth [10, 36]. As a result, the alveolar part of MS in the retroclined incisor group is longer or taller compared to those in the upright incisors’ group, as found in this study. MS elongates to meet with opposing teeth when mandible growth outpaced the upper jaw [37]. Hence, incisor inclination influences the morphology of MS as mentioned by other studies [1, 12, 13].
Our findings also show that alveolar bone at the B-point level was very narrow and constricted in patients with Class III skeletal bases who have high gonial angle. Furthermore, the alveolar bone surrounding lower incisors decreases in thickness as the gonial angle increases. This suggests that orthodontists must be extra careful when moving teeth in patients of the high gonial angle group, as there is higher risk of root movement which can lead to gingival recession, root resorption and bone fenestration [38].
In a study by Swasty et al. [16], males generally have significantly taller MS than females. Our current findings are also in line, where male MS is taller and narrower at the lingual basal part and B point area compared to female (P<0.006). Similar findings were also observed in other studies [3, 6, 11, 16, 39]. The differences in skeletal shape, including dental arches is often reported with significant differences noted [40, 41]. Bite forces, for example, is also reported to influence bone remodeling. Males have almost four times higher bite force than females, at 190 N and 50 N respectively [42].
In conclusion, the shape of MS in Class III skeletal base varied at the alveolar part, basal part or both. The alveolar part ranged from tall and narrow with inferiorly positioned B-point to short, wide with superiorly positioned B-point. The basal part ranged from narrow, elongated, and oval-shaped to wide, short, and bulbous-shaped. The factors identified to highly influence MS shape was gonial angle, followed by incisor inclination and sex.
Acknowledgements
The authors wish to thank Faculty of Dentistry, UKM for the support in funding this project under Geran Galakan Penyelidikan FGG (DD-2021-008).
Notes
References
1. Yamada C, Kitai N, Kakimoto N, Murakami S, Furukawa S, Takada K. 2007; Spatial relationships between the mandibular central incisor and associated alveolar bone in adults with mandibular prognathism. Angle Orthod. 77:766–72. DOI: 10.2319/072906-309. PMID: 17685772.
2. Choi YJ, Chung CJ, Kim KH. 2015; Periodontal consequences of mandibular incisor proclination during presurgical orthodontic treatment in Class III malocclusion patients. Angle Orthod. 85:427–33. DOI: 10.2319/021414-110.1. PMID: 25090134. PMCID: PMC8612433.
3. Lee KA, Kim MS, Hong JY, Lee JS, Choi SH, Chai JK, Jung UW. 2014; Anatomical topography of the mandibular symphysis in the Korean population: a computed tomography analysis. Clin Anat. 27:592–7. DOI: 10.1002/ca.22315. PMID: 24343797.
4. Chung CJ, Jung S, Baik HS. 2008; Morphological characteristics of the symphyseal region in adult skeletal Class III crossbite and openbite malocclusions. Angle Orthod. 78:38–43. DOI: 10.2319/101606-427.1. PMID: 18193946.
5. Guerino P, Marquezan M, Mezomo MB, Antunes KT, Grehs RA, Ferrazzo VA. 2017; Tomographic evaluation of the lower incisor's bone limits in mandibular symphysis of orthodontically untreated adults. Biomed Res Int. 2017:9103749. DOI: 10.1155/2017/9103749. PMID: 29181407. PMCID: PMC5664189.
6. Arruda KEM, Neto JV, de Araújo Almeida G. 2012; Assessment of the mandibular symphysis of Caucasian Brazilian adults with well-balanced faces and normal occlusion: the influence of gender and facial type. Dent Press J Orthod. 17:40–50. DOI: 10.1590/S2176-94512012000300012.
7. Gracco A, Luca L, Bongiorno MC, Siciliani G. 2010; Computed tomography evaluation of mandibular incisor bony support in untreated patients. Am J Orthod Dentofacial Orthop. 138:179–87. DOI: 10.1016/j.ajodo.2008.09.030. PMID: 20691359.
8. Handelman CS. 1996; The anterior alveolus: its importance in limiting orthodontic treatment and its influence on the occurrence of iatrogenic sequelae. Angle Orthod. 66:246. DOI: 10.1043/0003-3219(1996)066<0095:TAAIII>2.3.CO;2. PMID: 8712499.
9. Mangla R, Singh N, Dua V, Padmanabhan P, Khanna M. 2011; Evaluation of mandibular morphology in different facial types. Contemp Clin Dent. 2:200–6. DOI: 10.4103/0976-237X.86458. PMID: 22090764. PMCID: PMC3214527.
10. Molina-Berlanga N, Llopis-Perez J, Flores-Mir C, Puigdollers A. 2013; Lower incisor dentoalveolar compensation and symphysis dimensions among Class I and III malocclusion patients with different facial vertical skeletal patterns. Angle Orthod. 83:948–55. DOI: 10.2319/011913-48.1. PMID: 23758599. PMCID: PMC8722832.
11. Bangar C, Wagh S, Murthy K, Parhad S. 2015; Compensatory cephalometric changes in mandibular symphysis with different anteroposterior jaw relationships. Int J Oral Health Med Res. 2:3–7.
12. Nor MM, Manan M, Mohamed AM, Rosli TI, Hafiz A. 2020; Lower incisor inclination and bony support in class II skeletal pattern patients-CBCT study. J Res Med Dent Sci. 8:85–93.
13. Yu Q, Pan XG, Ji GP, Shen G. 2009; The association between lower incisal inclination and morphology of the supporting alveolar bone--a cone-beam CT study. Int J Oral Sci. 1:217–23. DOI: 10.4248/IJOS09047. PMID: 20690425. PMCID: PMC3733600.
14. Fukase H. 2007; Functional significance of bone distribution in the human mandibular symphysis. Anthropol Sci. 115:55–62. DOI: 10.1537/ase.060329.
15. Proffit WR, Fields HW. 1983; Occlusal forces in normal- and long-face children. J Dent Res. 62:571–4. DOI: 10.1177/00220345830620051301. PMID: 6573374.
16. Swasty D, Lee J, Huang JC, Maki K, Gansky SA, Hatcher D, Miller AJ. 2011; Cross-sectional human mandibular morphology as assessed in vivo by cone-beam computed tomography in patients with different vertical facial dimensions. Am J Orthod Dentofacial Orthop. 139(4 Suppl):e377–89. DOI: 10.1016/j.ajodo.2009.10.039. PMID: 21435546.
17. Maniyar M, Kalia A, Hegde A, Gautam RG, Mirdehghan N. 2014; Lower incisor dentoalveolar compensation and symphysis dimensions in class II and class III patients. Int J Dent Med Spec. 1:20–4. DOI: 10.5958/2394-4196.2014.00005.3.
18. Moshfeghi M, Nouri M, Mirbeigi S, Baghban AA. 2014; Correlation between symphyseal morphology and mandibular growth. Dent Res J (Isfahan). 11:375–9. DOI: 10.4103/1735-3327.135915. PMID: 25097649. PMCID: PMC4119372.
19. Aki T, Nanda RS, Currier GF, Nanda SK. 1994; Assessment of symphysis morphology as a predictor of the direction of mandibular growth. Am J Orthod Dentofacial Orthop. 106:60–9. DOI: 10.1016/S0889-5406(94)70022-2. PMID: 8017351.
20. Pan JY, Chou ST, Chang HP, Liu PH. 2006; Morphometric analysis of the mandible in subjects with Class III malocclusion. Kaohsiung J Med Sci. 22:331–8. DOI: 10.1016/S1607-551X(09)70319-5. PMID: 16849101.
21. Woon CK, Jamal NAA, Noor MNIM, Abdullah SM, Mohamed Ibrahim N, Norman NH, Alias A. 2019; Geometric morphometric analysis of malocclusion on lateral cephalograms in Malaysian population. Anat Cell Biol. 52:397–405. Erratum in: Anat Cell Biol 2020;53:378. DOI: 10.5115/acb.19.118. PMID: 31949978. PMCID: PMC6952698.
22. Rohlf FJ, Marcus LF. 1993; A revolution morphometrics. Trends Ecol Evol. 8:129–32. DOI: 10.1016/0169-5347(93)90024-J. PMID: 21236128.
23. Mitteroecker P, Gunz P. 2009; Advances in geometric morphometrics. Evol Biol. 36:235–47. DOI: 10.1007/s11692-009-9055-x.
24. Cocos A, Halazonetis DJ. 2017; Craniofacial shape differs in patients with tooth agenesis: geometric morphometric analysis. Eur J Orthod. 39:345–51. DOI: 10.1093/ejo/cjw049. PMID: 27464525.
25. Katsadouris A, Halazonetis DJ. 2017; Geometric morphometric analysis of craniofacial growth between the ages of 12 and 14 in normal humans. Eur J Orthod. 39:386–94. DOI: 10.1093/ejo/cjw070. PMID: 27940444.
26. Cardini A, Seetah K, Barker G. 2015; How many specimens do I need? Sampling error in geometric morphometrics: testing the sensitivity of means and variances in simple randomized selection experiments. Zoomorphology. 134:149–63. DOI: 10.1007/s00435-015-0253-z.
27. Izard G. 1927; The goniomandibular angle in dentofacial orthopedia. Int J Orthod Oral Surg Radiogr. 13:578–81. DOI: 10.1016/S0099-6963(27)90110-0.
28. Rohlf FJ. 2005. tpsDig, digitize landmarks and outlines, version 2.05. State University of New York at Stony Brook;Stony Brook: DOI: 10.1016/s0099-6963(27)90110-0.
29. Bookstein FL. Bookstein FL, editor. 1992. Chapter 3: landmarks. Morphometric Tools for Landmark Data: Geometry and Biology. Cambridge University Press;Cambridge: p. 55–87. DOI: 10.1017/CBO9780511573064.004.
30. Klingenberg CP. 2011; MorphoJ: an integrated software package for geometric morphometrics. Mol Ecol Resour. 11:353–7. DOI: 10.1111/j.1755-0998.2010.02924.x. PMID: 21429143.
31. Zelditch M, Swiderski D, Sheets H, Fink W. Zelditch M, Swiderski D, Sheets H, Fink W, editors. 2004. Simple size and shape variables: Bookstein shape coordinates. Geometric Morphometrics for Biologists: A Primer. Academic Press;London: p. 51–72. DOI: 10.1016/B978-012778460-1/50005-3.
32. García-Morales P, Buschang PH, Throckmorton GS, English JD. 2003; Maximum bite force, muscle efficiency and mechanical advantage in children with vertical growth patterns. Eur J Orthod. 25:265–72. DOI: 10.1093/ejo/25.3.265. PMID: 12831216.
33. Grünheid T, Langenbach GE, Korfage JA, Zentner A, van Eijden TM. 2009; The adaptive response of jaw muscles to varying functional demands. Eur J Orthod. 31:596–612. DOI: 10.1093/ejo/cjp093. PMID: 19656804.
34. Ueda HM, Miyamoto K, Saifuddin M, Ishizuka Y, Tanne K. 2000; Masticatory muscle activity in children and adults with different facial types. Am J Orthod Dentofacial Orthop. 118:63–8. DOI: 10.1067/mod.2000.99142. PMID: 10893474.
35. Sella-Tunis T, Pokhojaev A, Sarig R, O'Higgins P, May H. 2018; Human mandibular shape is associated with masticatory muscle force. Sci Rep. 8:6042. DOI: 10.1038/s41598-018-24293-3. PMID: 29662127. PMCID: PMC5902585.
36. Karlsen AT. 1995; Craniofacial growth differences between low and high MP-SN angle males: a longitudinal study. Angle Orthod. 65:341–50. DOI: 10.1043/0003-3219(1995)065<0341:CGDBLA>2.0.CO;2. PMID: 8526293.
37. Richardson ME. 2002; Late lower arch crowding: the aetiology reviewed. Dent Update. 29:234–8. DOI: 10.12968/denu.2002.29.5.234. PMID: 12096382.
38. Wennström JL. 1996; Mucogingival considerations in orthodontic treatment. Semin Orthod. 2:46–54. DOI: 10.1016/S1073-8746(96)80039-9. PMID: 9161283.
39. Uysal T, Yagci A, Ozer T, Veli I, Ozturk A. 2012; Mandibular anterior bony support and incisor crowding: is there a relationship? Am J Orthod Dentofacial Orthop. 142:645–53. DOI: 10.1016/j.ajodo.2012.05.017. PMID: 23116505.
40. Dempsey PJ, Townsend GC, Martin NG, Neale MC. 1995; Genetic covariance structure of incisor crown size in twins. J Dent Res. 74:1389–98. DOI: 10.1177/00220345950740071101. PMID: 7560390.
41. Ursi WJ, Trotman CA, McNamara JA Jr, Behrents RG. 1993; Sexual dimorphism in normal craniofacial growth. Angle Orthod. 63:47–56. DOI: 10.1043/0003-3219(1993)063<0047:SDINCG>2.0.CO;2. PMID: 8507031.
42. Osborn J, Mao J. 1993; A thin bite-force transducer with three-dimensional capabilities reveals a consistent change in bite-force direction during human jaw-muscle endurance tests. Arch Oral Biol. 38:139–44. DOI: 10.1016/0003-9969(93)90198-U. PMID: 8476343.
Fig. 2
Procrustes superimposition of 254 samples. The blue circle encompassing landmarks (numbered in red) indicate landmark variation of the 254 samples.
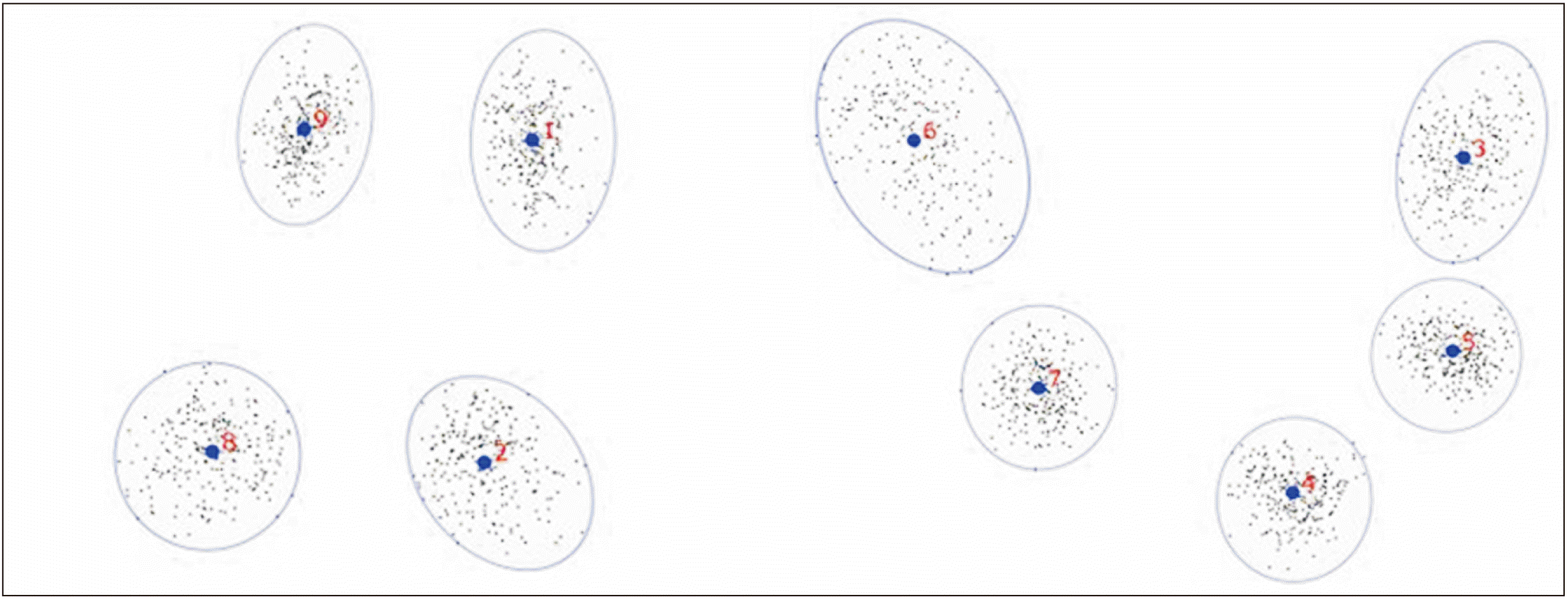
Fig. 3
Shapes changes associated with the first three principal components (PCs), representing extremes PC scores along each PC axis. Light blue: mean shape of the samples. Dark blue: shape variation.
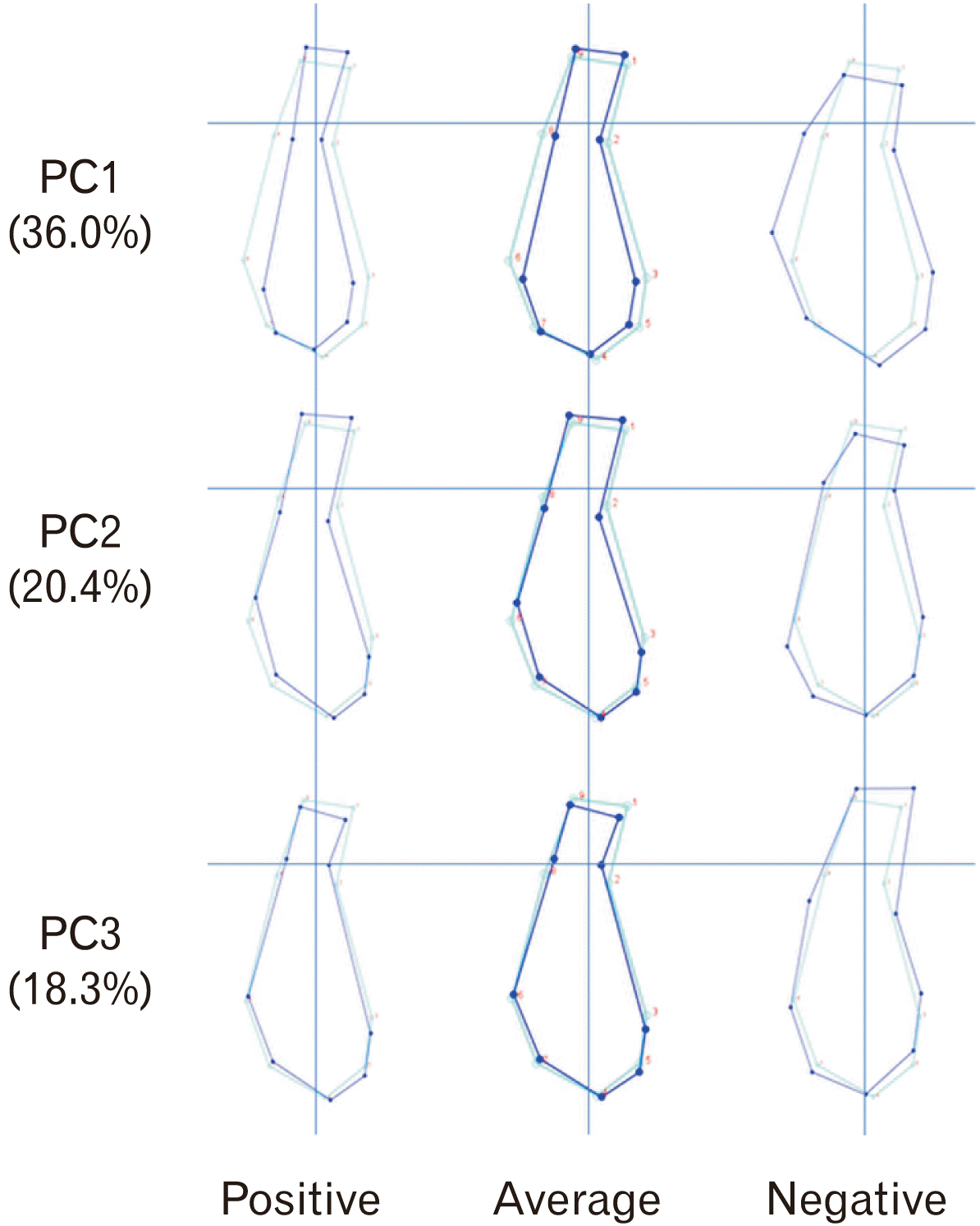
Fig. 4
Scatter plot of canonical variate (CV)1 vs. CV2. The groups are defined by gonial angle. The groups are defined by gonial angle. Light blue: mean shape of the samples. Dark blue: shape variation.
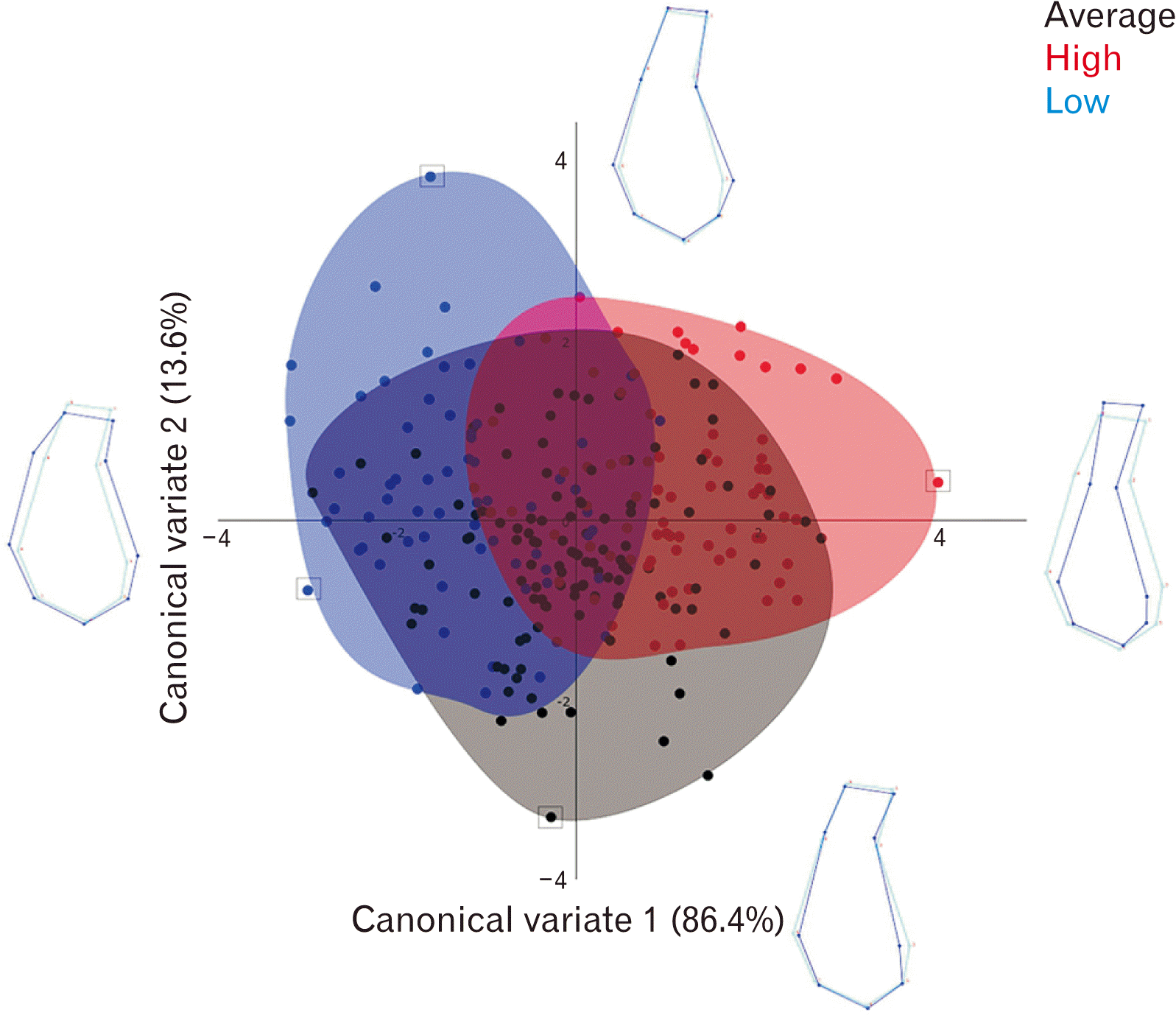
Table 1
Description of landmarks on mandibular symphysis




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download