1. New York Heart Association. 1994. Criteria Committee. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. 9th ed. Little Brown;Boston:
2. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021; 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 42:3599–726. DOI:
10.1093/eurheartj/ehab368. PMID:
34447992.
3. Goldman L, Cook EF, Mitchell N, Flatley M, Sherman H, Cohn PF. 1982; Pitfalls in the serial assessment of cardiac functional status. How a reduction in 'ordinary' activity may reduce the apparent degree of cardiac compromise and give a misleading impression of improvement. J Chronic Dis. 35:763–71. DOI:
10.1016/0021-9681(82)90087-X. PMID:
7119078.
4. Goldman L, Hashimoto B, Cook EF, Loscalzo A. 1981; Comparative reproducibility and validity of systems for assessing cardiovascular functional class: advantages of a new specific activity scale. Circulation. 64:1227–34. DOI:
10.1161/01.CIR.64.6.1227. PMID:
7296795.

5. Caraballo C, Desai NR, Mulder H, Alhanti B, Wilson FP, Fiuzat M, et al. 2019; Clinical implications of the New York Heart Association classification. J Am Heart Assoc. 8:e014240. DOI:
10.1161/JAHA.119.014240. PMID:
31771438. PMCID:
PMC6912957.

6. Raphael C, Briscoe C, Davies J, Ian Whinnett Z, Manisty C, Sutton R, et al. 2007; Limitations of the New York Heart Association functional classification system and self-reported walking distances in chronic heart failure. Heart. 93:476–82. DOI:
10.1136/hrt.2006.089656. PMID:
17005715. PMCID:
PMC1861501.

7. Spinar J, Spinarova L, Malek F, Ludka O, Krejci J, Ostadal P, et al. 2019; Prognostic value of NT-proBNP added to clinical parameters to predict two-year prognosis of chronic heart failure patients with mid-range and reduced ejection fraction-A report from FAR NHL prospective registry. PLoS One. 14:e0214363. DOI:
10.1371/journal.pone.0214363. PMID:
30913251. PMCID:
PMC6435170.
8. Gardner RS, Özalp F, Murday AJ, Robb SD, McDonagh TA. 2003; N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. 24:1735–43. DOI:
10.1016/j.ehj.2003.07.005. PMID:
14522568.

9. Kang SH, Park JJ, Choi DJ, Yoon CH, Oh IY, Kang SM, et al. 2015; Prognostic value of NT-proBNP in heart failure with preserved versus reduced EF. Heart. 101:1881–8. DOI:
10.1136/heartjnl-2015-307782. PMID:
26319121.

10. Poelzl G, Trenkler C, Kliebhan J, Wuertinger P, Seger C, Kaser S, et al. 2014; FGF23 is associated with disease severity and prognosis in chronic heart failure. Eur J Clin Invest. 44:1150–8. DOI:
10.1111/eci.12349. PMID:
25294008.

11. Koller L, Kleber ME, Brandenburg VM, Goliasch G, Richter B, Sulzgruber P, et al. 2015; Fibroblast growth factor 23 is an independent and specific predictor of mortality in patients with heart failure and reduced ejection fraction. Circ Heart Fail. 8:1059–67. DOI:
10.1161/CIRCHEARTFAILURE.115.002341. PMID:
26273098.

12. Roy C, Lejeune S, Slimani A, de Meester C, Ahn As SA, Rousseau MF, et al. 2020; Fibroblast growth factor 23: a biomarker of fibrosis and prognosis in heart failure with preserved ejection fraction. ESC Heart Fail. 7:2494–507. DOI:
10.1002/ehf2.12816. PMID:
32578967. PMCID:
PMC7524237.

13. Mendez Fernandez AB, Ferrero-Gregori A, Garcia-Osuna A, Mirabet-Perez S, Pirla-Buxo MJ, Cinca-Cuscullola J, et al. 2020; Growth differentiation factor 15 as mortality predictor in heart failure patients with non-reduced ejection fraction. ESC Heart Fail. 7:2223–9. DOI:
10.1002/ehf2.12621. PMID:
32589369. PMCID:
PMC7524215.

14. Gaggin HK, Szymonifka J, Bhardwaj A, Belcher A, de Berardinis B, Motiwala S, et al. 2014; Head-to-head comparison of serial soluble ST2, growth differentiation factor-15, and highly-sensitive troponin T measurements in patients with chronic heart failure. JACC Heart Fail. 2:65–72. DOI:
10.1016/j.jchf.2013.10.005. PMID:
24622120.

15. Kempf T, von Haehling S, Peter T, Allhoff T, Cicoira M, Doehner W, et al. 2007; Prognostic utility of growth differentiation factor-15 in patients with chronic heart failure. J Am Coll Cardiol. 50:1054–60. DOI:
10.1016/j.jacc.2007.04.091. PMID:
17825714.

16. Emdin M, Aimo A, Vergaro G, Bayes-Genis A, Lupón J, Latini R, et al. 2018; sST2 predicts outcome in chronic heart failure beyond NT-proBNP and high-sensitivity troponin T. J Am Coll Cardiol. 72:2309–20. DOI:
10.1016/j.jacc.2018.08.2165. PMID:
30384887.
17. Bouwens E, Brankovic M, Mouthaan H, Baart S, Rizopoulos D, van Boven N, et al. 2019; Temporal patterns of 14 blood biomarker candidates of cardiac remodeling in relation to prognosis of patients with chronic heart failure-The Bio- SH i FT study. J Am Heart Assoc. 8:e009555. DOI:
10.1161/JAHA.118.009555. PMID:
30760105. PMCID:
PMC6405680.

18. Masson S, Agabiti N, Vago T, Miceli M, Mayer F, Letizia T, et al. 2015; The fibroblast growth factor-23 and vitamin D emerge as nontraditional risk factors and may affect cardiovascular risk. J Intern Med. 277:318–30. DOI:
10.1111/joim.12232. PMID:
24620922.

20. Sun RR, Lu L, Liu M, Cao Y, Li XC, Liu H, et al. 2014; Biomarkers and heart disease. Eur Rev Med Pharmacol Sci. 18:2927–35.
21. Yap J, Lim FY, Gao F, Teo LL, Lam CSP, Yeo KK. 2015; Correlation of the New York Heart Association classification and the 6‐minute walk distance: A systematic review. Clin Cardiol. 38:621–8. DOI:
10.1002/clc.22468. PMID:
26442458. PMCID:
PMC6490728.

22. Felker GM, Whellan D, Kraus WE, Clare R, Zannad F, Donahue M, et al. 2009; N-terminal pro-brain natriuretic peptide and exercise capacity in chronic heart failure: data from the Heart Failure and a Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION) study. Am Heart J. 158:S37–44. DOI:
10.1016/j.ahj.2009.07.011. PMID:
19782787. PMCID:
PMC3748954.

23. Bozkurt B, Coats AJ, Tsutsui H, Abdelhamid M, Adamopoulos S, Albert N, et al. 2021; Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J Card Fail. 27:387–413. DOI:
10.1016/j.cardfail.2021.01.022.

24. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF, Dokainish H, Edvardsen T, et al. 2016; Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 29:277–314. DOI:
10.1016/j.echo.2016.01.011. PMID:
27037982.

25. Guyatt GH, Sullivan MJ, Thompson PJ, Fallen EL, Pugsley SO, Taylor DW, et al. 1985; The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J. 132:919–23.
26. Demers C, McKelvie RS, Negassa A, Yusuf S. RESOLVD Pilot Study Investigators. 2001; Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 142:698–703. DOI:
10.1067/mhj.2001.118468. PMID:
11579362.

27. Masson S, Latini R, Anand IS, Vago T, Angelici L, Barlera S, et al. 2006; Direct comparison of B-type natriuretic peptide (BNP) and amino-terminal proBNP in a large population of patients with chronic and symptomatic heart failure: the Valsartan Heart Failure (Val-HeFT) data. Clin Chem. 52:1528–38. DOI:
10.1373/clinchem.2006.069575. PMID:
16777915.

29. Kuster N, Huet F, Dupuy AM, Akodad M, Battistella P, Agullo A, et al. 2020; Multimarker approach including CRP, sST2 and GDF-15 for prognostic stratification in stable heart failure. ESC Heart Fail. 7:2230–9. DOI:
10.1002/ehf2.12680. PMID:
32649062. PMCID:
PMC7524044.

30. Grande D, Leone M, Rizzo C, Terlizzese P, Parisi G, Gioia MI, et al. 2017; A multiparametric approach based on NT-proBNP, ST2, and galectin3 for stratifying one year prognosis of chronic heart failure outpatients. J Cardiovasc Dev Dis. 4:9. DOI:
10.3390/jcdd4030009. PMID:
29367540. PMCID:
PMC5715710.

31. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, et al. 2013; Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. 34:1404–13. DOI:
10.1093/eurheartj/ehs337. PMID:
23095984.

32. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. 2006; The Seattle heart failure model: prediction of survival in heart failure. Circulation. 113:1424–33. DOI:
10.1161/CIRCULATIONAHA.105.584102. PMID:
16534009.
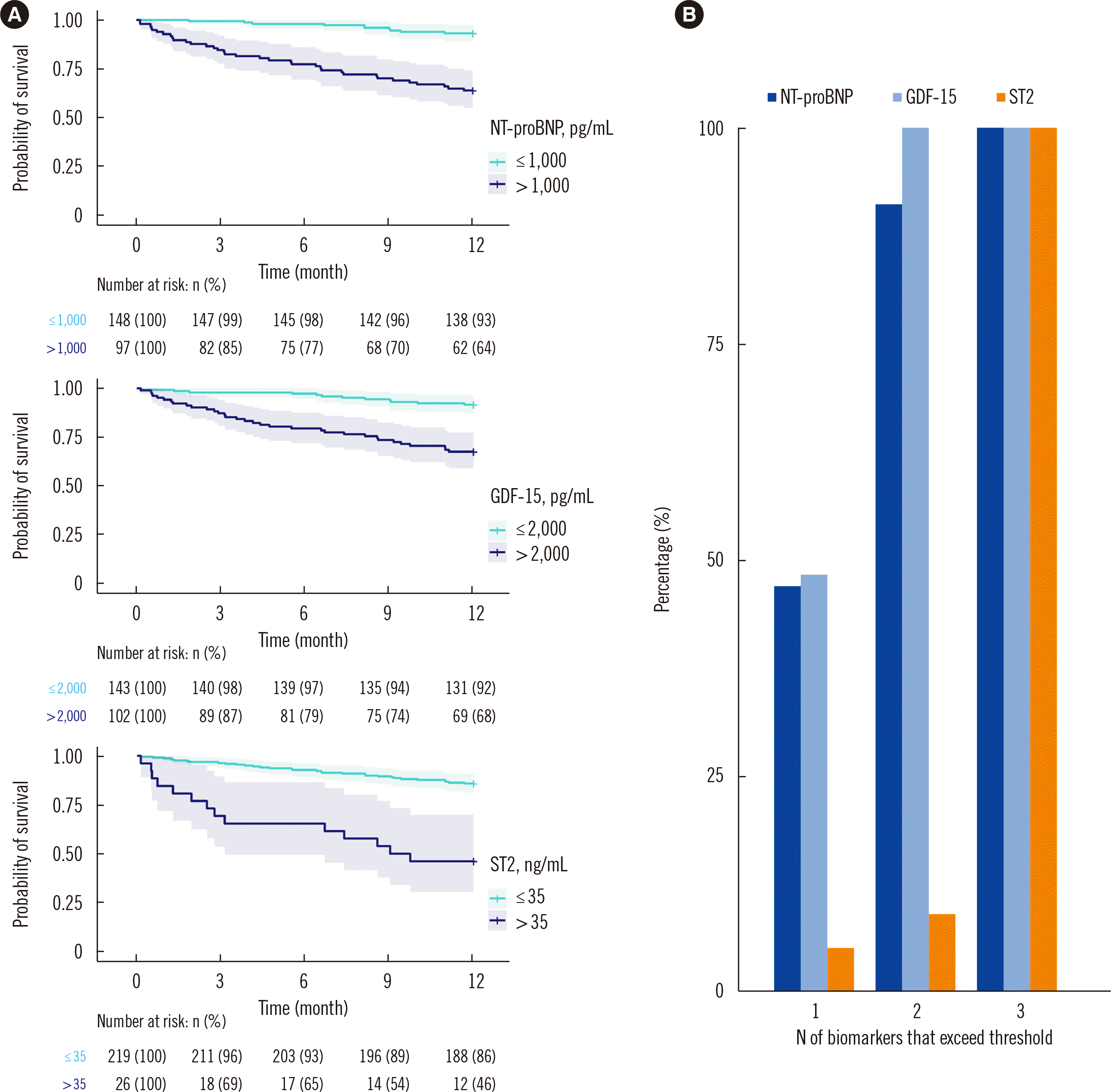
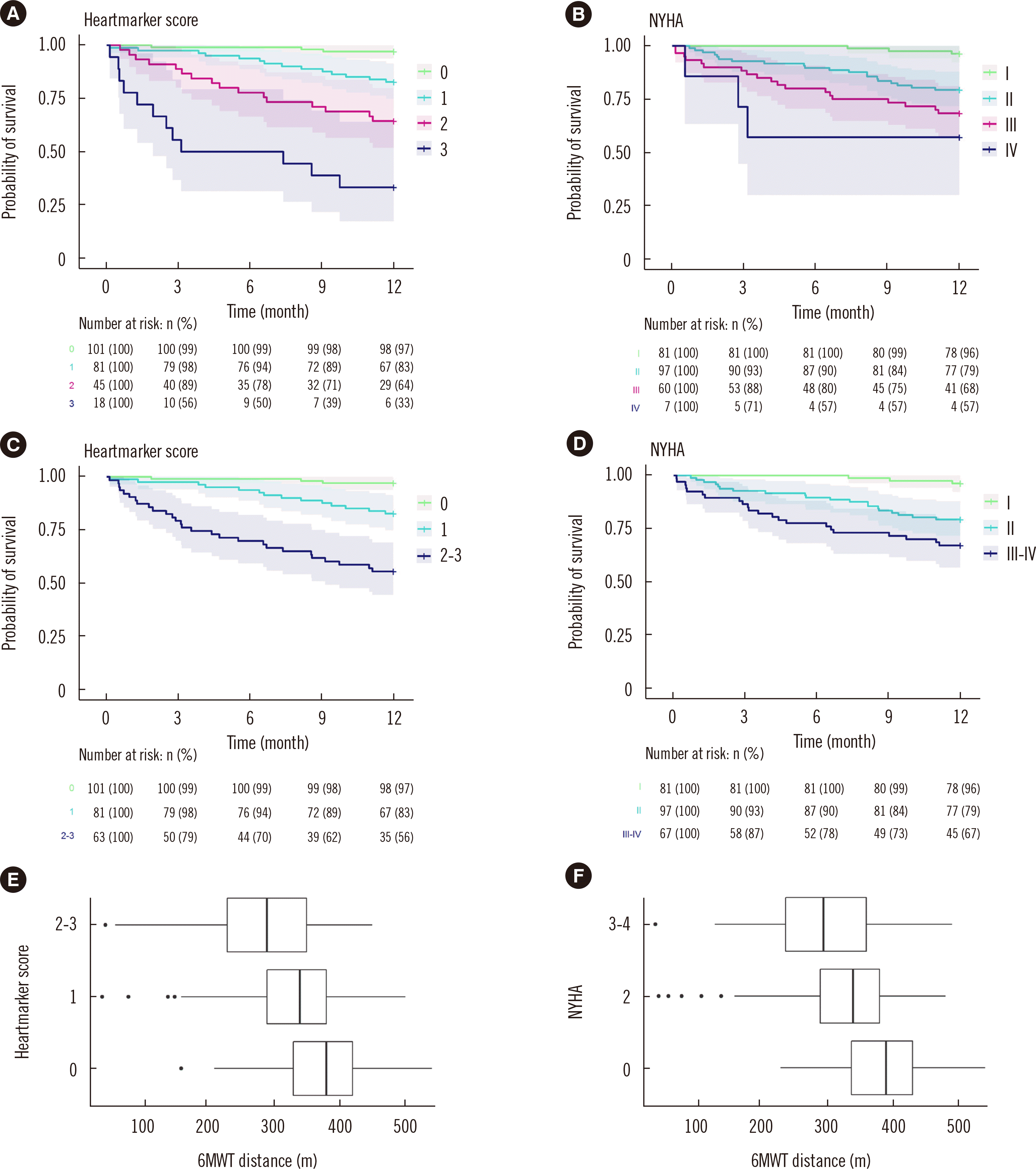




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download