INTRODUCTION
The relationship between cholesterol concentration and atherosclerotic cardiovascular disease (ASCVD) has been studied for several years. LDL, the dominant form of cholesterol, is considered an important risk factor for ASCVD. However, the risk of ASCVD remains even after lowering LDL cholesterol (LDL-C) to a normal concentration [
1-
3]. Other forms of cholesterol are associated with ASCVD, which are included in current global cholesterol guidelines [
4,
5]. This increased the importance of non-HDL cholesterol (non-HDL-C) and apolipoprotein B (ApoB). Non-HDL-C concentrations are calculated by subtracting the concentration of HDL-C from that of total cholesterol, comprising very low-density lipoprotein cholesterol (VLDL-C), intermediate-density lipoprotein cholesterol (IDL-C), remnant particles, lipoprotein (a) [Lp (a)], and LDL-C [
6]. Not only LDL-C, all of them is known to play an important role in the atherosclerosis evolution. ApoB is the main protein found in VLDL-C, IDL-C, LDL-C, and Lp (a). Each of these lipoprotein particles includes a single ApoB protein; thus, measurement of ApoB concentrations indicates the number of ApoB-containing lipoproteins [
7].
In most individuals, LDL-C, non-HDL-C, and ApoB concentrations correlate well; however, there is some discordance in individuals with hypertriglyceridemia, diabetes mellitus (DM), and metabolic syndrome [
8]. Non-HDL-C and ApoB will increase with more cholesterol other than LDL-C, and ApoB will increase when the number of particles is high even if the cholesterol level is normal. However, discordance analysis has consistently shown that ASCVD risk follows lipid particle number and its surrogates rather than lipid cholesterol concentration [
8]. It is known that the patients with DM are seemed to have smaller and more LDL particles than the other patients although LDL-C is not increased [
9], which means higher ApoB concentration in the DM patients. According to 2019 ESC/ECS guidelines, ApoB, more than non-HDL-C, is recommended for risk assessment, particularly in people with high triglyceride levels, DM, obesity, or very low LDL-C concentrations [
5].
Although studies have shown the superiority of non-HDL-C and ApoB as lipid markers for ASCVD [
10-
12], they are considered secondary markers [
4,
5]. Both are recommended when LDL-C is very low or only in specific patient groups. In this study, as primary marker, LDL-C, non-HDL-C, and ApoB concentrations in participants of the Korean Genome and Epidemiology Study (KoGES) were compared with respect to the risk of cardiovascular disease. To our knowledge, this is the first Korean prospective cohort study exploring the association between ASCVD and lipid markers, including ApoB.
RESULTS
Among the 5,872 participants, 252 (4.3%) had ASCVD during the 8-year follow-up period. The median (interquartile range [IQR]) follow-up period was 95 (93–97) months. Baseline characteristics, including sex, age, hypertension, DM status, smoking status, CKD status, family history of premature ASCVD, and LDL-C, non-HDL-C, and ApoB concentrations, are presented in
Table 1. All variables were available for 5,872 participants, except for ApoB, which was available for 5,160 participants. The prevalence of hypertension, DM, and CKD was significantly higher in the cardiovascular event group than in the non-cardiovascular event group. The cardiovascular event group was older and had higher non-HDL-C and ApoB concentrations (
Table 1).
Table 1
Baseline characteristics of participants
|
Characteristic |
8-year cardiovascular disease event |
P*
|
|
|
Yes (N=252) |
No (N=5,620) |
|
Sex, N (%) |
|
|
0.103 |
|
Male |
131 (52.0) |
2,627 (46.7) |
|
|
Female |
121 (48.0) |
2,993 (53.3) |
|
|
Age, median (IQR) |
62 (52–67) |
55 (50–64) |
< 0.001 |
|
Hypertension, N (%) |
83 (32.9) |
1,272 (22.6) |
< 0.001 |
|
DM, N (%) |
38 (15.1) |
564 (10.0) |
0.010 |
|
Current smoking, N (%) |
47 (18.7) |
949 (16.9) |
0.452 |
|
CKD, N (%) |
21 (8.3) |
275 (4.9) |
0.015 |
|
Family history, N (%) |
15 (6.0) |
225 (4.0) |
0.125 |
|
Lipid markers, median (IQR) |
|
|
|
|
LDL-C, mmol/L |
3.24 (2.73–3.90) |
3.19 (2.65–3.71) |
0.058 |
|
Non-HDL-C, mmol/L |
3.97 (3.44–4.70) |
3.88 (3.31–4.45) |
0.003 |
|
ApoB (μmol/L; N= 5,160) |
2.16 (1.85–2.53) |
2.05 (1.75–2.39) |
0.001 |

Univariate Cox regression analysis was performed to determine the time-dependent effects (
Supplemental Data Table S2). Participants with hypertension, DM, or CKD at baseline had a significantly higher risk for an 8-year cardiovascular event. Male sex, older age, and higher lipid marker concentrations were associated with a higher risk of 8-year cardiovascular event development. Smoking status and a family history of premature ASCVD were not associated with an 8-year cardiovascular event.
Multivariate Cox regression analysis was performed separately for the three lipid markers (LDL-C, non-HDL-C, and ApoB) and other confounding variables, including sex, age, hypertension, DM, CKD, smoking status, and family history of premature ASCVD (
Supplemental Data Table S3). Males with DM at baseline had a significantly higher risk of an 8-year cardiovascular event. Male sex, older age, and higher lipid marker concentrations were associated with a higher risk of an 8-year cardiovascular event. Smoking status, hypertension, and CKD were not associated with the risk of 8-year cardiovascular event development. The aHRs for ASCVD per 1-SD increase in each of the three lipid marker concentrations are presented in
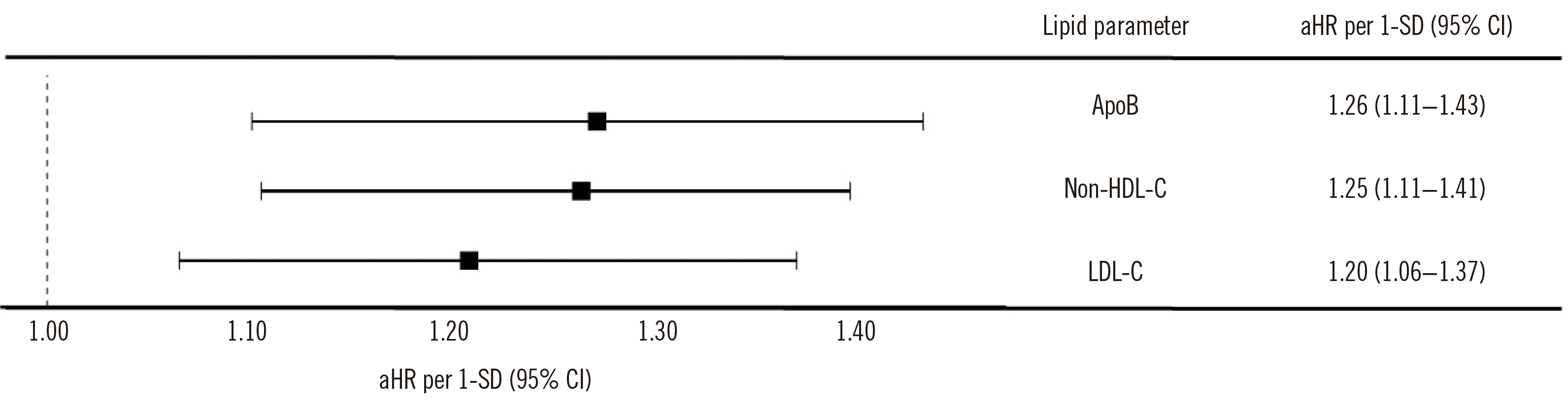
Fig. 2. Among the three lipid markers, ApoB showed the highest aHR per 1-SD of 1.26 (95% CI, 1.11–1.43), followed by non-HDL-C at 1.25 (95% CI, 1.11–1.41) and LDL-C at 1.20 (95% CI, 1.06–1.37). When grouped by tertiles of each lipid marker, the aHRs for ASCVD in the highest tertiles were 1.51 for LDL-C, 1.47 for non-HDL-C, and 1.53 for ApoB compared to those in the lowest tertile (
Supplemental Data Table S4). The risk of ASCVD was similar between the middle and lowest tertiles for all three lipid markers.
Fig. 2
aHRs for atherosclerotic cardiovascular disease per 1-standard deviation increase in lipid marker concentrations.
Abbreviations: aHR, adjusted hazard ratio; ApoB, apolipoprotein B; Non-HDL-C, non-HDL cholesterol; LDL-C, LDL cholesterol; CI, confidence interval.

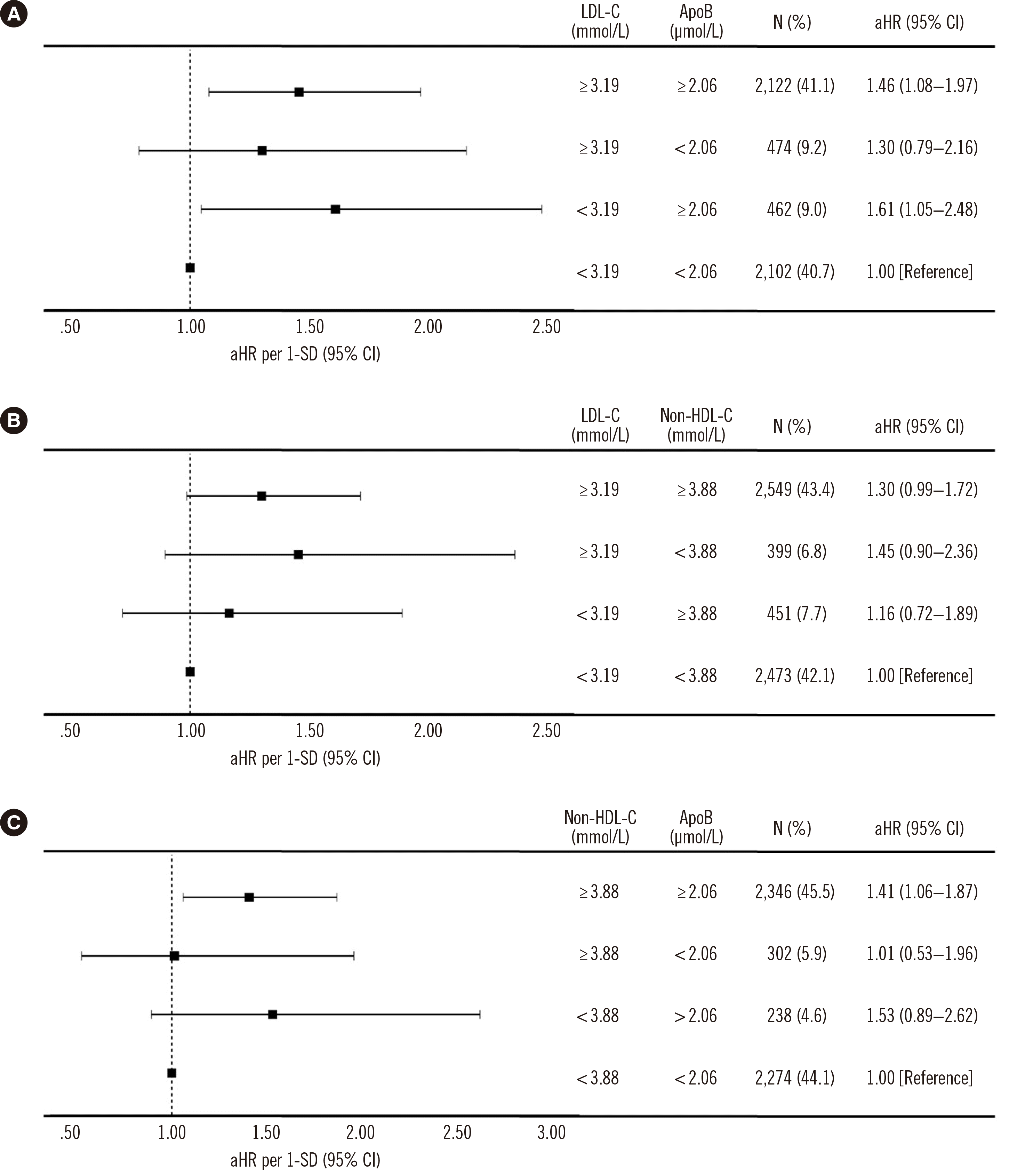
Participants were also grouped according to the median values of the three lipid markers to confirm which markers are most suitable for predicting ASCVD. The aHR of each group was calculated by adjusting for the effects of other variables (
Fig. 3). Based on the median values, the aHR for ASCVD was 1.30 (95% CI, 0.79–2.16) in the group with high LDL-C and low ApoB concentrations and was 1.61 (95% CI, 1.05–2.48) in the group with low LDL-C and high ApoB concentrations (
Fig. 3A). The aHR in the group with high LDL-C and low non-HDL-C concentrations was 1.45 (95% CI, 0.90–2.36), and that in the group with low LDL-C and high non-HDL-C concentrations was 1.16 (95% CI, 0.72–1.89) (
Fig. 3B). Finally, the aHR in the group with high non-HDL-C and low ApoB concentrations was 1.01 (95% CI, 0.53–1.96), and that in the group with low non-HDL-C and high ApoB concentrations was 1.53 (95% CI, 0.89–2.62) (
Fig. 3C).
Fig. 3
aHRs of participants grouped by (A) LDL-C and ApoB, (B) LDL-C and non-HDL-C, (C) non-HDL-C and ApoB.
Abbreviations: aHR, adjusted hazard ratio; ApoB, apolipoprotein B; CI, confidence interval; non-HDL-C, non-HDL cholesterol; LDL-C, LDL cholesterol.

DISCUSSION
Non-HDL-C and ApoB are important markers for the management of blood cholesterol, as they represent LDL-C and other minor cholesterols that cause ASCVD. However, these are treated as secondary markers, and LDL-C remains the main marker of dyslipidemia in clinical practice.
We analyzed prospective cohort data to compare the predictive ability of the three lipid markers (non-HDL-C, LDL-C, and ApoB) for 8-year ASCVD risk. Recent clinical guidelines consider most individuals with DM to be at high or very high risk of ASCVD, regardless of their lipid concentrations [
4,
5]. To adjust for confounding factors, including DM, we performed a multivariate analysis including all factors known to be associated with ASCVD. When considering other variables, LDL-C, non-HDL-C, and ApoB concentrations were significantly associated with a higher risk of 8-year cardiovascular event development. The aHR per 1-SD was the highest for ApoB, followed by non-HDL-C and LDL-C. When grouped by median values of two lipid markers, the aHR of the group with low LDL-C and high ApoB concentrations was higher than that of the reference group (low LDL-C and low ApoB concentrations). This reflects the importance of ApoB concentrations in predicting ASCVD, regardless of LDL-C concentrations. The ApoB test is recommended for individuals with apparently normal LDL-C concentrations if they have other risk factors for ASCVD.
In univariate Cox regression analysis, hypertension and CKD were associated with a higher risk of 8-year cardiovascular event development, while a family history of premature ASCVD was not. The opposite results were obtained in the multivariate Cox regression analysis. The discordant results may be due to the association among the variables. Hypertension and CKD are closely related to age (
P<0.001, data not shown); when analyzed together, the risk of ASCVD increased, although statistical significance was not reached (
Supplemental Data Table S3). Meta-analyses, including more independent cohort studies, may be helpful in verifying the association of these variables with ASCVD. Smoking is also a known risk factor for ASCVD, but we could not determine its significance in our data. This may be because current smoking status does not perfectly reflect an individual’s smoking history; for instance, although the participant may have been a smoker at the time of the survey, their pack-years may have been very short. Conversely, a long-term smoker might have quit smoking at the time of the survey. Participants also may not have answered truthfully. For predicting ASCVD, other factors such as smoking intensity may be more suitable than smoking status [
14]. Since only smoking status was included in our analysis, we could not perform further evaluations of smoking history.
Studies have compared these three lipid markers in ASCVD [
10-
12,
15-
17]; however, identifying the most potent marker of ASCVD is challenging. Some studies indicated that ApoB may not be superior to non-HDL-C and LDL-C [
15,
16], whereas other recent studies indicated ApoB as a superior marker for predicting ASCVD. Johannesen,
et al. [
11] followed 13,015 individuals treated with a statin for 8 years; the HRs of the high ApoB and low LDL-C group were 1.21 (95% CI, 1.07–1.36) for all-cause mortality and 1.49 (95% CI, 1.15–1.92) for myocardial infarction compared with those of the low ApoB and low LDL-C groups. Corresponding values for the high non-HDL-C and low LDL-C groups were 1.18 (95% CI, 1.02–1.36) and 1.78 (95% CI, 1.35–
2.34), respectively. However, high LDL-C and low ApoB or non-HDL-C were not significantly associated with all-cause mortality or myocardial infarction. Marston,
et al. [
17] conducted a prospective cohort study that included 389,529 participants in the primary prevention group and 40,430 participants in the statin-treated group. When the three lipid markers were evaluated together, only ApoB was associated with myocardial infarction in the primary prevention (aHR: 1.27; 95% CI, 1.15–1.40) and statin treatment groups. A meta-analysis including 233,455 participants showed that the relative risk ratios for cardiovascular event were 1.43 (95% CI, 1.31–1.51), 1.34 (95% CI, 1.24–1.44), and 1.25 (95% CI, 1.18–1.33) for ApoB, non-HDL-C, and LDL-C, respectively [
10]. Another meta-analysis comparing risk reduction after statin therapy showed that the mean coronary heart disease risk reduction per change in ApoB concentration was 21.6% (95% CI, 12.0%–31.2%) greater than that observed per change in LDL-C concentration (
P<0.001) and was 24.3% (95% CI, 22.4%–26.2%) greater than that observed per change in non-HDL-C concentration (
P<0.001) [
12]. In our study, ApoB showed the highest aHR per 1-SD of 1.26 (95% CI, 1.11–1.43), non-HDL-C showed an aHR of 1.25 (95% CI, 1.11–1.41), and LDL-C showed the lowest aHR of 1.20 (95% CI, 1.06–1.37) after adjusting for sex, age, hypertension, DM, current smoking, and family history of premature ASCVD and CKD.
This study has some limitations. First, the data were dependent on the participants’ responses to the survey; therefore, inaccurate information could have been included, such as incorrect diagnosis or incorrect onset age. Second, the definition of coronary artery disease was not clear because the KoGES surveyor did not provide a clear definition. Presence of disease according to an International Classification of Diseases (ICD) code in the medical records would be more appropriate. Finally, we could not determine which lipid marker was the best for predicting ASCVD using these cohort data alone. As shown in
Fig. 3, ApoB was a significant predictor even for individuals with low LDL-C concentrations, whereas non-HDL-C concentrations were not significant. From an analytical viewpoint, the non-HDL-C concentration is dependent on the calculation method, and the assumed total error of total cholesterol concentration plus HDL-C concentration is larger than that of ApoB [
18]. There may be a difference between LDL-C calculated by the classical Friedewald formula and directly measured LDL-C [
19], which may have affected our results.
In conclusion, ApoB, non-HDL-C, and LDL-C are independent risk factors for ASCVD. Increases in the aHR per 1-SD for ASCVD were more greatly affected by ApoB, non-HDL-C, and LDL-C, in that order, based on KoGES cohort data. Participants with low LDL-C but high ApoB concentrations showed an increased risk of ASCVD. In individuals with ASCVD risk factors, even those presenting with normal LDL-C concentrations, measuring ApoB can provide useful information for better assessment of ASCVD risk.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download