Abstract
Deceased donor liver transplantation (DDLT) using donations after brain death (DBDs) has been widely performed in Korea. However, to date, there is no report regarding donation after circulatory death (DCD) category III. A 56-year-old male patient diagnosed with hepatitis B virus-associated liver cirrhosis underwent DDLT using DCD category III. The recipient’s recovery was uneventful, and he was discharged on postoperative day 37. Currently, the patient is alive, with no complications 20 months after transplantation. This case suggests that DCD with LT is both feasible and safe. Further studies are required to validate this finding.
Go to : 
Although liver transplantation (LT) is theoretically the best treatment option for patients with end-stage liver disease, donor organs remain scarce. To overcome this problem, the concept of donation after circulatory death (DCD) has emerged as an alternative option to donation after brain death (DBD) to expand the shortage of the donor pool.
Recent studies have reported good outcomes after DCD LT, with low biliary complication rates and graft and patient survival rates comparable to those of DBD LT [1]. However, due to concerns regarding poor outcomes in recipients and legal issues, LT using DCD (especially DCD category III) has not been implemented in South Korea. In the current report, we present the first case of a patient with hepatitis B virus (HBV)-associated liver cirrhosis who underwent successful deceased donor LT (DDLT) using DCD category III.
Go to : 
Institutional review board approval for this study was waived because this was a case report of a single patient that did not include protected health information, data analysis, or testing of a hypothesis and was de-identified. Informed consent forms were provided to the patient based on the Declaration of Helsinki, and the patient provided voluntary consent.
A 52-year-old male patient was diagnosed with cerebral hemorrhage on June 23, 2020. On the same day, he was registered as a potential brain-dead donor in the Korean Network for Organ Sharing (KONOS) database. However, five consecutive electroencephalography (EEG) tests at intervals of 2 to 3 days did not show flat EEGs. Even in this situation, the family members very strongly hoped to donate the patient’s organs. The KONOS accepted this exceptional condition through an internal meeting, and the patient was finally approved to donate an organ in DCD category III (awaiting cardiac arrest).
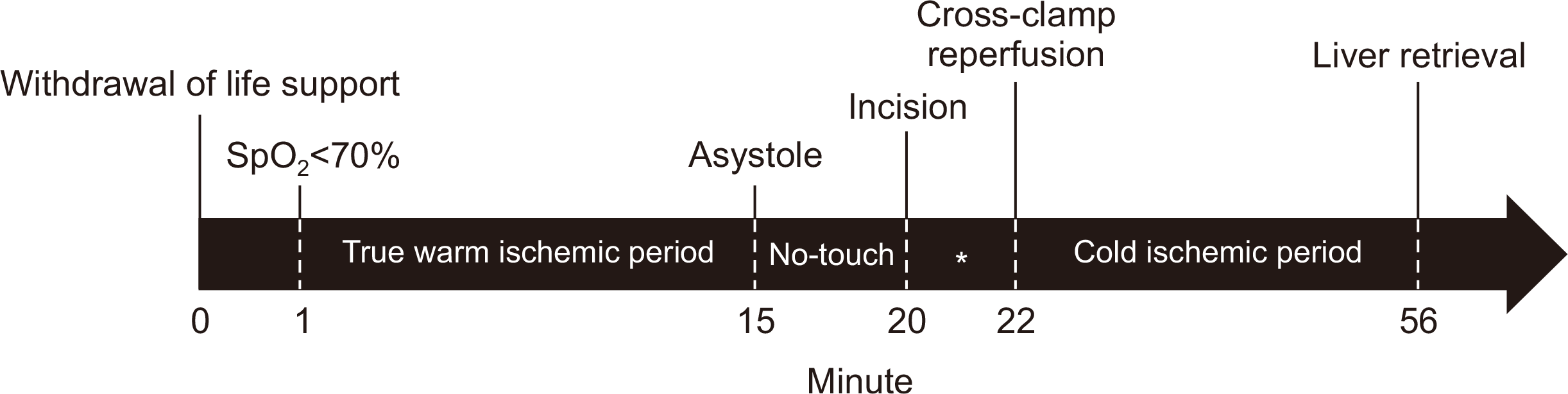
The patient underwent surgery on July 3, 2020. The timeline of DCD is shown in Fig. 1. The specific time sequence of the donation process has been published previously [2]. After the withdrawal of life-sustaining therapy (WLST), which includes termination of cardiopulmonary support and all essential medications, oxygen saturation decreased to 70% in 1 minute. Subsequently, asystole was confirmed. Following a 5-minute “no-touch time,” organ procurement surgery was initiated. The liver was retrieved using a super-rapid technique. The functional (true) warm ischemic time was 14 minutes, and the total warm ischemic time was 21 minutes.
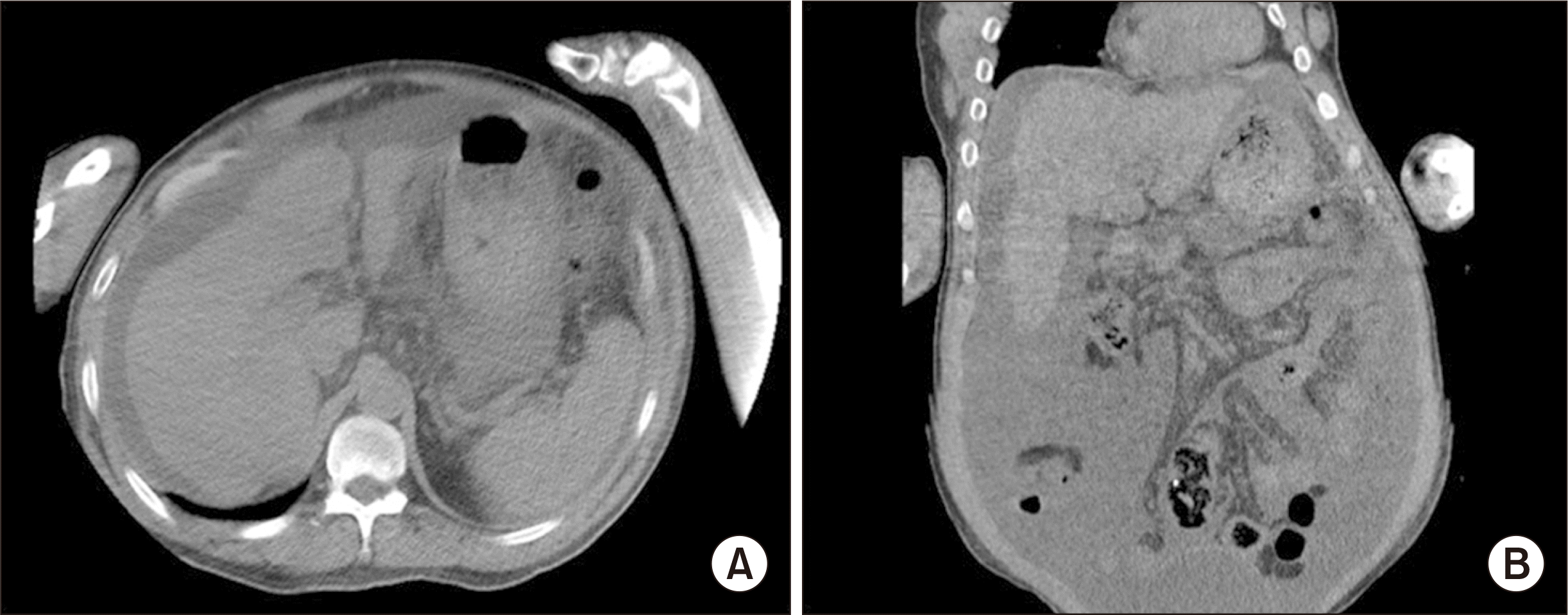
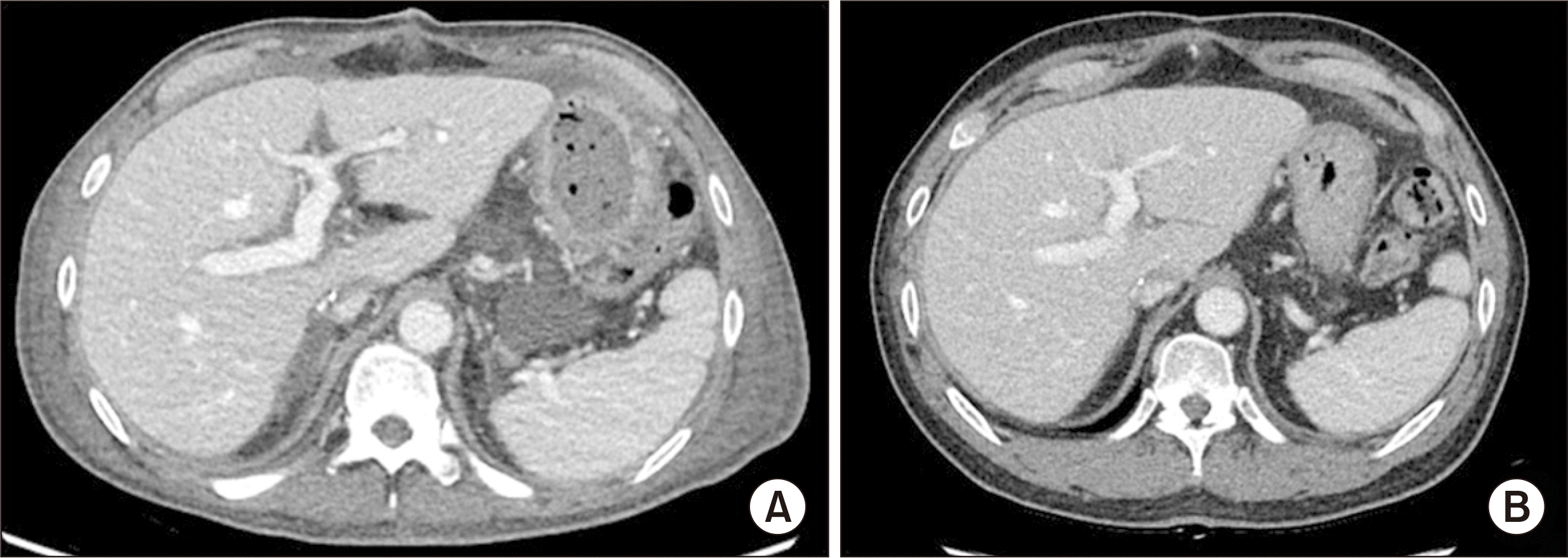
A 56-year-old male patient diagnosed with HBV-associated liver cirrhosis was selected as the recipient of the liver according to the routine process of the model for end-stage liver disease (MELD) scoring system in Korea. The MELD score was 29. Preoperative imaging revealed liver cirrhosis with a large amount of ascites (Fig. 2). Recipient surgery was performed uneventfully, according to the standard DDLT procedure. The total operative time was 395 minutes. The estimated intraoperative blood loss was 2,580 mL. The pathological diagnosis was liver cirrhosis associated with HBV. The postoperative course was uneventful. The patient’s liver function normalized on postoperative day 6. He was transferred to the general ward on postoperative day 15. The patient recovered routinely without any specific problems, except for ascites. The patient was discharged 37 days postoperatively without any complications. At 20 months after DCD LT, the patient was alive with a well-functioning graft (Fig. 3).
Go to : 
Over the past few decades, the outcomes of LT have dramatically improved owing to the gradual optimization of surgical techniques and perioperative management, as well as the appropriate use of immunosuppressants [3,4]. However, with an increasing number of patients on the LT waiting list, a profound organ shortage has been identified as a significant limitation in the field of transplantation. Accordingly, to increase the supply of available donors, various strategies have been proposed, such as using old and marginal living donors [5] and the development of alternative techniques, including liver graft splitting [6] and domino LT [7].
From this perspective, the concept of DCD has emerged as an essential source of deceased organ donors to use expanded criteria organs. In 1995, DCD was initially classified into four categories at an international workshop held in Maastricht [8]. According to the recently revised Maastricht classification of DCD, DCD category III (controlled DCD) refers to organ donation from a patient who had cardiac arrest following planned WLST but was not considered brain-dead [9]. In Western countries, where there is a relatively high number of brain-dead donors, LT using DCD has been mainly performed from category III donors in a controlled environment. Conversely, considering the small number of deceased donors (25% of all LT cases) in South Korea [4], activation of DCD category III can be an alternative option to solve the organ transplantation demand-supply mismatch.
To date, in South Korea, DCD category III is not permitted for organ donation by law due to ethical issues. However, in this case, the potential donor did not show flat EEGs on five consecutive examinations. Along with the family’s strong willingness to donate, attempts to expand into challenging new areas by the Korean Society for Transplantation eventually led to the success of this case. To the best of our knowledge, this case is the first successful report of LT from DCD category III in South Korea.
Meanwhile, in this case, before procurement, the lung and liver transplant team discussed and simulated the optimal sequential scenario for minimizing the time from incision to cross-clamping, because this time (asterisk in Fig. 1) is the only adjustable period to shorten donor warm ischemia [10]. The warm and cold ischemic times are well-known risk factors that affect graft viability [10]. Therefore, minimizing the operative time is highly recommended. With the “super-rapid” technique, which was used in this case, the time from incision to cross-clamping was only 2 minutes. Moreover, various factors influencing graft survival after LT from DCD, such as a younger donor [11], use of thrombolytic agents [1], premortem heparin administration [12], and ex vivo machine perfusion [13], have been investigated to achieve successful outcomes in DCD LT. In this case, premortem heparin administration was performed to optimize the liver perfusion before organ harvest.
In early experiences, DCD grafts were reported to have higher rates of biliary complications and poorer graft survival than traditional DBD [14]. However, as effective therapeutic modalities have been optimized and surgical experience has been accumulated over time, these problems have steadily decreased. Park et al. [15] recently proposed a new Korean guideline for organ donation based on DCD category III. Based on this report, we hope that DCD will be more actively applied in South Korea.
In conclusion, the present case demonstrates that LT with DCD category III can be completed with satisfactory outcomes. Thus, DCD category III should be considered as a feasible treatment option to reduce the number of patients on the waiting list. Therefore, further experience is required.
Go to : 
ACKNOWLEDGMENTS
Funding/Support
This study was supported by research grant from the Korean Society for Transplantation (2022-00-02003-018).
Author Contributions
Conceptualization: IK, JGL. Visualization: IK, JL. Writing–original draft: IK. Writing–review & editing: IK, JL, JGL.
Go to : 
REFERENCES
1. Bohorquez H, Seal JB, Cohen AJ, Kressel A, Bugeaud E, Bruce DS, et al. 2017; Safety and outcomes in 100 consecutive donation after circulatory death liver transplants using a protocol that includes thrombolytic therapy. Am J Transplant. 17:2155–64. DOI: 10.1111/ajt.14261. PMID: 28276658.
2. Jeong E, Oh J, Lee Y, Lee J. 2020; The first donation after circulatory death following withdrawal of life-sustaining treatment in Korea. Korean J Transplant. 34(Suppl 1):S30. DOI: 10.4285/ATW2020.OR-1066.
3. Kang I, Lee JG, Choi SH, Kim HJ, Han DH, Choi GH, et al. 2021; Impact of everolimus on survival after liver transplantation for hepatocellular carcinoma. Clin Mol Hepatol. 27:589–602. DOI: 10.3350/cmh.2021.0038. PMID: 34293849. PMCID: PMC8524068.
4. Choi HJ. 2022; Current status and outcome of liver transplantation in South Korea. Clin Mol Hepatol. 28:117–9. DOI: 10.3350/cmh.2021.0381. PMID: 34875151. PMCID: PMC8755474.
5. Goldaracena N, Cullen JM, Kim DS, Ekser B, Halazun KJ. Expanding the donor pool for liver transplantation with marginal donors. Int J Surg. 2020; 82 Suppl:30–5. DOI: 10.1016/j.ijsu.2020.05.024. PMID: 32422385.
6. Renz JF, Yersiz H, Reichert PR, Hisatake GM, Farmer DG, Emond JC, et al. 2003; Split-liver transplantation: a review. Am J Transplant. 3:1323–35. DOI: 10.1046/j.1600-6135.2003.00254.x. PMID: 14525591.
7. Marques HP, Barros I, Li J, Murad SD, di Benedetto F. Current update in domino liver transplantation. Int J Surg. 2020; 82S:163–8. DOI: 10.1016/j.ijsu.2020.03.017. PMID: 32244002.
8. Kootstra G, Daemen JH, Oomen AP. 1995; Categories of non-heart-beating donors. Transplant Proc. 27:2893–4. PMID: 7482956.
9. Thuong M, Ruiz A, Evrard P, Kuiper M, Boffa C, Akhtar MZ, et al. 2016; New classification of donation after circulatory death donors definitions and terminology. Transpl Int. 29:749–59. DOI: 10.1111/tri.12776. PMID: 26991858.
10. Hashimoto K. 2020; Liver graft from donation after circulatory death donor: real practice to improve graft viability. Clin Mol Hepatol. 26:401–10. DOI: 10.3350/cmh.2020.0072. PMID: 32646199. PMCID: PMC7641554.
11. Mathur AK, Heimbach J, Steffick DE, Sonnenday CJ, Goodrich NP, Merion RM. 2010; Donation after cardiac death liver transplantation: predictors of outcome. Am J Transplant. 10:2512–9. DOI: 10.1111/j.1600-6143.2010.03293.x. PMID: 20977642.
12. Narvaez JR, Nie J, Noyes K, Kayler LK. 2020; Transplant outcomes of donation after circulatory death livers recovered with versus without premortem heparin administration. Liver Transpl. 26:247–55. DOI: 10.1002/lt.25685. PMID: 31755633.
13. Schlegel A, Muller X, Kalisvaart M, Muellhaupt B, Perera MT, Isaac JR, et al. 2019; Outcomes of DCD liver transplantation using organs treated by hypothermic oxygenated perfusion before implantation. J Hepatol. 70:50–7. DOI: 10.1016/j.jhep.2018.10.005. PMID: 30342115.
14. Feng S, Goodrich NP, Bragg-Gresham JL, Dykstra DM, Punch JD, DebRoy MA, et al. 2006; Characteristics associated with liver graft failure: the concept of a donor risk index. Am J Transplant. 6:783–90. DOI: 10.1111/j.1600-6143.2006.01242.x. PMID: 16539636.
15. Park H, Jung ES, Oh JS, Lee YM, Lee JM. 2021; Organ donation after controlled circulatory death (Maastricht classification III) following the withdrawal of life-sustaining treatment in Korea: a suggested guideline. Korean J Transplant. 35:71–6. DOI: 10.4285/kjt.21.0004. PMID: 35769520. PMCID: PMC9235338.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download