This study was approved by the Institutional Review Board of Korea University Medical Center (IRB No. 2022AN0185), which waived the requirement for informed consent due to the retrospective nature of this study.
A 61-year-old female patient with chronic kidney disease (CKD) due to diabetes mellitus and hypertension-induced nephropathy received a deceased donor kidney transplant in March 2020 from a 61-year-old male with 1/8 human leukocyte antigen mismatch. Basiliximab was used as the induction therapy. Tacrolimus, mycophenolate mofetil (MMF), and methylprednisolone comprised the maintenance immunosuppressive regimen. She was discharged 10 days after with normal kidney function (serum creatinine [sCr], 0.74 mg/dL). Four months later, on postoperative day 122, she was transferred from a local hospital for the exacerbation of general weakness and diarrhea after 3 days of conservative care. Upon her arrival, we noticed a high level of sCr (1.5 mg/dL) and a significant decrease in urine output. Her laboratory results indicated significant hemolysis, with a hemoglobin level of 7.0 g/dL a platelet count of 20 ×10
3/μL, and an LDH level of 3,207 IU/L. In addition, a peripheral blood smear showed schistocytes. A disintegrin and metalloproteinase with thrombospondin type 1 motif, member 13 (ADAMTS-13) activity was within the normal range (
Table 1). Stool culture was negative. Kidney biopsy was performed to rule out acute rejection, and the biopsy results showed severe TMA without evidence of acute rejection (
Fig. 1). There was no microvascular inflammation, such as glomerular inflammatory infiltrate or peritubular capillary inflammatory infiltrate, and C4d immunohistochemical staining was negative. Thus, according to the 2019 Banff classification, acute antibody-mediated rejection was excluded. Under the impression of aHUS, we replaced tacrolimus with sirolimus and immediately started plasmapheresis. Hemodialysis was also initiated for anuria. At the same time, we requested approval of eculizumab from the Health Insurance Review and Assessment Service (HIRA). As the cost of eculizumab is very high, in order to be covered by public insurance, it is necessary to obtain approval from the HIRA before its used in Korea. Eculizumab was considered as a kidney graft rescue therapy since plasmapheresis did not effectively decrease the patient’s sCr levels and anuria continued despite plasmapheresis (
Table 2). Vaccination against meningococcal disease was performed on admission day 9 and the use of eculizumab was approved by HIRA on admission day 10. Immediately after approval for use, eculizumab (900 mg) was administered weekly for 4 weeks. An additional 600 mg of eculizumab was administered on the day of plasmapheresis. Since the patient’s laboratory parameters gradually improved, hemodialysis and plasmapheresis were ceased on admission day 37. After that, 1,200 mg of eculizumab was administered biweekly two more times until HIRA recommended cessation (
Fig. 2). At the time of discharge, the patient’s platelet count had recovered to 236 ×10
3/μL, the LDH level had decreased to 1,379 IU/L, and sCr had decreased to 2.05 mg/dL (
Fig. 3). We continued to use MMF and steroid therapy, including sirolimus, which replaced tacrolimus, as maintenance immunosuppressants. The trough level of sirolimus was around 4 to 6 mg/dL. Based on the patient’s laboratory data and clinical signs, we determined that she had recovered from aHUS without significant adverse effects except for an asymptomatic urinary tract infection. The most recent laboratory results at 2 years after KT showed a platelet count of 335 ×10
3/μL, sCr level of 1.32 mg/dL, and LDH level of 420 IU/L.
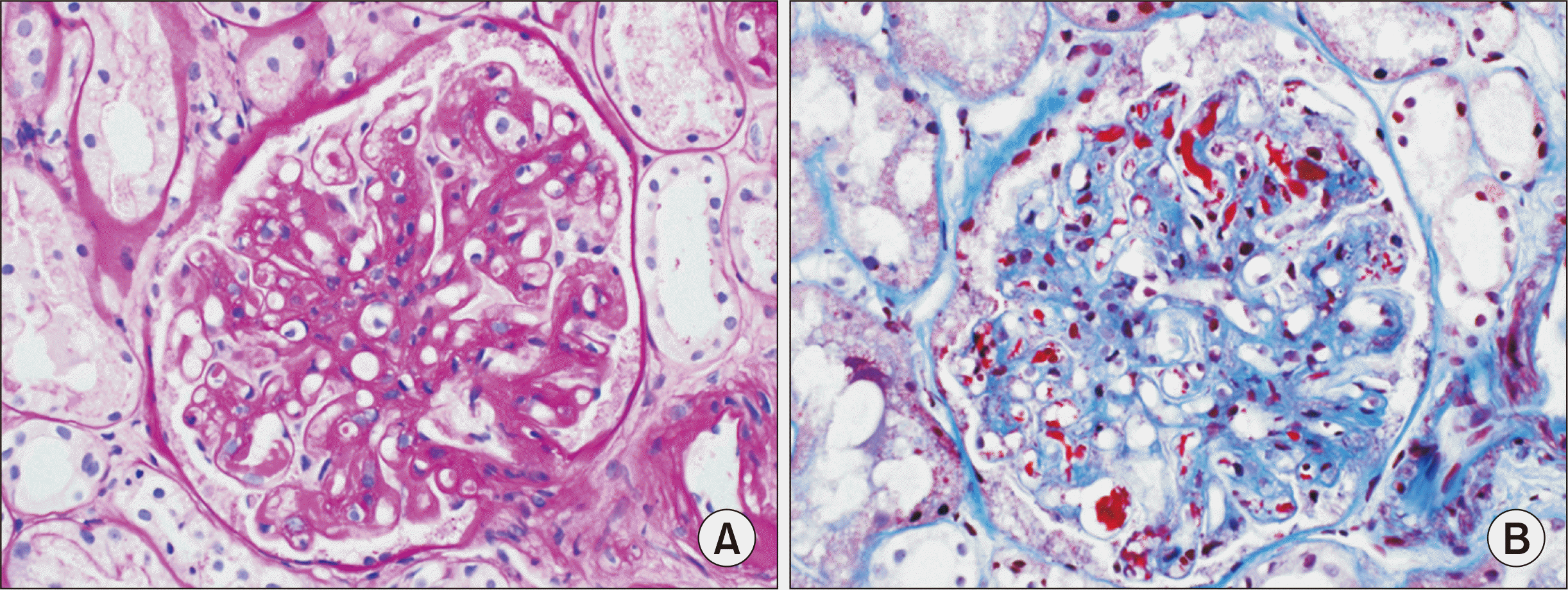 | Fig. 1(A) Kidney biopsy on postoperative day 123 (admission day 2) showing severe thrombotic microangiopathy (TMA; high-resolution pictures showing endothelial swelling, glomerular fibrin thrombi, and glomerulus with entrapped fragmented red blood cells). There was no evidence of active antibody-mediated rejection, such as glomerular inflammatory infiltrate or peritubular capillary inflammatory infiltrate. Periodic acid-Schiff (PAS) stain, ×400. (B) Kidney biopsy on postoperative day 123 (admission day 2) showing severe TMA (high-resolution pictures showing endothelial swelling, glomerular fibrin thrombi, and glomerulus with entrapped fragmented red blood cells). There was no evidence of active antibody mediated rejection, such as glomerular inflammatory infiltrate or peritubular capillary inflammatory infiltrate. Masson’s trichrome stain, ×400. 
|
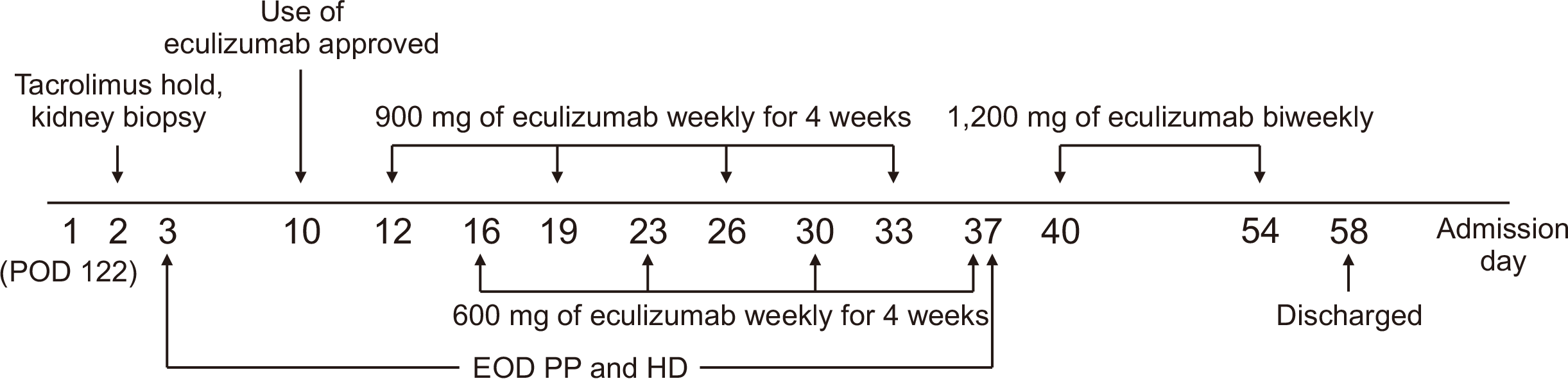 | Fig. 2Treatment summary after admission. Plasmapheresis was performed four times before eculizumab treatment and 13 times during eculizumab treatment. POD, postoperative day; EOD, every other day; PP, plasmapheresis; HD, hemodialysis. 
|
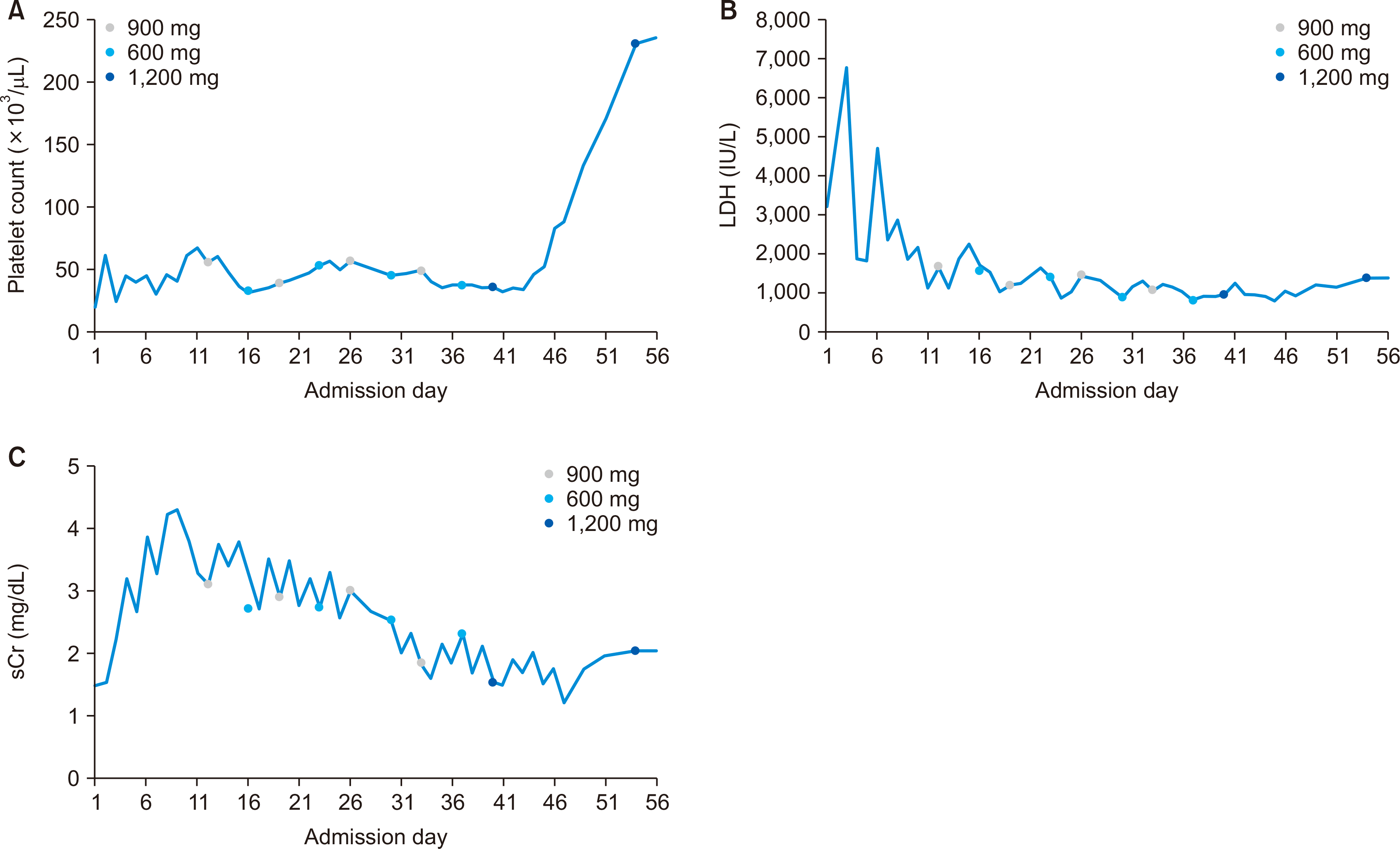 | Fig. 3Improvements in (A) platelet count, (B) lactate dehydrogenase (LDH), and (C) serum creatinine (sCr) during 8 weeks of eculizumab treatment. 
|
Table 1
Results of laboratory tests on the day of arrival
|
Test |
Result |
|
Hb (g/dL) |
7.0 |
|
Reticulocyte count (% of RBCs) |
1.3 |
|
Haptoglobin (mg/dL) |
<5 |
|
Platelet (×103/μL) |
20 |
|
Immature platelet fraction (%)a)
|
15.8 |
|
BUN/Cr (mg/dL) |
53.6/1.50 |
|
LDH (IU/L) |
3,207 |
|
Albumin (g/dL) |
2.7 |
|
Proteinuria on dipstick |
3+ |
|
Microscopic red blood cell (/HPF) |
10–29 |
|
PB smear |
Schistocyte (+++) |
|
Stool culture (O–157, Shigella, Salmonella, Vibrio, Yersinia, Campylobacter) |
Negative |
|
CH50 (U/mL) |
35 |
|
C3 (mg/dL) |
51.1 |
|
C4 (mg/dL) |
<8 |
|
Coombs test |
Negative |
|
ADAMTS-13 activity (ELISA) |
62.8% (normal, 50%–160%) |

Table 2
Changes of daily urine output
|
Admission day |
1 |
2 |
3 |
4 |
5–12 |
13–32 |
33–44 |
45–57 |
|
Total urine output (mL) |
1,220 |
1,070 |
150 |
20 |
0 |
100–300 |
>500 |
>1,000 |








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download