Abstract
Background
The use of sutures as ligatures has proven to be safe and reliable for the control of lymphatic vessels. The electrothermal bipolar vessel sealer (EBVS) is a relatively new type of device that can be used to seal lymphatics. We conducted a study to evaluate the safety and efficacy of EBVS for preparation of the recipient vessel during renal transplantation.
Methods
In this prospective randomized controlled study, EBVS (Medtronic) was compared with conventional ligature for the control of perivascular lymphatics in kidney transplant recipients. A total of 52 kidney transplant recipients were randomly assigned to two groups. In group 1, EBVS was used to control perivascular lymphatics, while conventional silk ligatures were used in group 2. Demographic characteristics, as well as preoperative, perioperative, and postoperative variables, were noted and compared between the groups.
Results
The mean recipient vessel preparation time was 8.3±1.9 minutes in group 1 and 14.5±4 minutes in group 2 (P<0.001). The mean anastomosis time was 28.2±5.4 minutes in group 1 and 28.2±4.2 minutes in group 2 (P=1.000). The mean estimated blood loss was 101.54±44.60 mL in group 1 and 125.19±74.17 mL in group 2 (P=0.270), and the mean drain output was 51.42 mL per day and 57.50 mL per day in groups 1 and 2, respectively (P=0.590).
Renal transplantation has become the mainstay and preferred treatment for patients of all ages with end-stage renal disease (ESRD) [1]. Various techniques are available for kidney transplantation, including open, laparoscopic, and robotic transplant. Open kidney transplantation remains the gold-standard option [2]. Kidney transplantation can be performed via an extraperitoneal approach or a transperitoneal approach. An extraperitoneal approach is always preferred in adults because it limits potential gastrointestinal complications and facilitates the confinement of potential surgical collections, such as blood and urinary leakage, to the retroperitoneum [3]. During kidney transplantation, the most important step in recipient vessel preparation is dissection of the iliac vessels with meticulous control of the perivascular lymphatics. Unlike blood, lymph does not contain clotting factors, so all lymphatics must be controlled precisely to avoid a very high risk of lymphocele formation [4]. Newer energy sources have been assessed by multiple researchers regarding their safety and efficacy for control of lymphatics compared to conventional ligature. Several devices that can be used for adequate lymphatic vessel closure have been presented to eliminate the need for clips and sutures [5,6]. The use of ligatures has proven to be safe and reliable for the control of lymphatics. The electrothermal bipolar vessel sealer (EBVS) is a relatively new type of bipolar energy device that can be used to seal the lymphatics as effectively as ligatures, but more quickly and easily. The safety and efficacy of EBVS have been well studied, but its use in renal transplantation is still limited [7]. Therefore, we conducted a study to evaluate the safety and efficacy of EBVS for preparation of the recipient vessel during renal transplant with particular focus on the incidence of posttransplant lymphocele. In this study, we compared silk ligature to the LigaSure (Medtronic, Dublin, Ireland) vessel sealing system for perivascular lymphatic control during recipient vessel preparation. In kidney transplantation, lymphatic fluid may leak from two sources: the kidney hilar lymphatics or lymphatics around the dissected recipient vessels. We desired to compare the outcomes of two methods of controlling the lymphatics around the iliac vessels. The lymphatics of the renal hilum were controlled similarly in both groups, by silk suture ligation during bench preparation of the kidney.
The study procedure aligned with the ethical standards for human experimentation as specified in the Declaration of Helsinki, and approval was obtained from the Institutional Ethics Committee of Institute of Kidney Disease and Research Centre (No. 17MAY2016). All patients were included in the study after providing informed written consent.
A prospective randomized controlled study was performed comparing EBVS (LigaSure; Medtronic) and conventional ligature for the control of perivascular lymphatics in kidney transplant recipients. The primary objective of the study was to compare the effectiveness of EBVS and ligature in controlling perivascular lymphatics. The secondary objectives were to assess recipient vessel preparation time and compare the groups with regard to complications, such as infection rate, and overall outcomes.
The study was conducted in the Department of Urology at the Institute of Kidney Diseases and Research Centre and the Dr. H. L. Trivedi Institute of Transplantation Sciences in Ahmedabad, Gujarat, India between March 2016 and February 2018. Any open renal transplant recipients of either sex and of any age with either a living or deceased donor were included in the study. All cases of dual kidney transplant, renal transplant in which vascular anastomosis was performed with vessels other than the external iliac, repeat renal transplant in the same anatomical space as before, and intraperitoneal graft placement were excluded from the study. Assuming a coefficient of variation of 65%, a confidence interval of 95%, and a power of 80%, the total sample size required was 52, with 26 patients in each group. For randomization, a sequence of 52 random numbers was generated using computer software, and 26 open renal transplant patients were randomly assigned to each group. In group 1, EBVS was used to control the perivascular lymphatics (Fig. 1), while in group 2, conventional 4.0 silk ligature was used for that purpose. After recruiting the subjects and receiving their consent, the sample was allocated between the two groups based on the sequence of randomly-assigned numbers.
The analysis included the profiling of patients regarding various demographic, clinical and laboratory parameters. Quantitative data were presented as means and standard deviations. Qualitative/categorical data were presented as absolute numbers and proportions. For nominal variables, the chi-square test or Fisher’s exact test was used to test associations depending on the normalcy of the sample. The Student t-test was used to compare the quantitative outcome parameters. A P-value of <0.05 was considered to indicate statistical significance, and IBM SPSS ver. 24.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis.
A thorough clinical evaluation was done as per the study protocol in all cases. A detailed history of each patient was taken with respect to age, sex, symptomatology, cause of renal failure, preoperative urine output, mode of dialysis, type of transplant, living or deceased donor, personal history, past urinary tract infections, and any history of renal transplant or any other surgical intervention. Each patient underwent all routine investigations and imaging. Both surgical approaches for lymphatic control were assessed and compared based on demographic data, clinical characteristics, and perioperative and postoperative parameters, and the safety and morbidity of each approach were judged. The perioperative variables noted are shown in Table 1. All vascular anastomoses were performed by an experienced surgeon with adequate experience in renal transplantation. All recipient vessels were prepared by an experienced surgeon. A double-J stent and drain were inserted following the institutional protocol if decided by the operating surgeon intraoperatively. Per the protocol, the stent and drain were inserted in cases where ureteric reimplant was difficult, the bladder mucosa was unhealthy, or generalized oozing was present.
A total of 52 renal transplant recipients were divided randomly into two study groups by method used for the control of the lymphatic channels. Group 1 included 26 cases in which the LigaSure (Medtronic) device was used, while group 2 included 26 cases in which silk 4.0 ligature was used. In group 1, the mean age was 36.2±14.1 years, while in group 2 it was 35.5±13.4 years (P=0.865). Among the 26 patients, group 1 included 19 (73.1%) male and seven (23.9%) female patients, for a male-to-female ratio of 2.7:1. Group 2 included 21 (80.8%) male and five (19.2%) female patients, for a male-to-female ratio of 4.2:1. Male predominance was noted in both groups. The body mass index was higher in group 1 (25.7±3.9 kg/m2) than in group 2 (22.8±3.7 kg/m2), which constituted a statistically significant difference (P=0.007).
The preoperative mean hemoglobin level was 10.5 gm/dL in group 1 and 10.7 gm/dL in group 2, constituting statistical similarity between groups (P=0.545). Table 2 presents the causes of ESRD in the study population. In most cases, ESRD was caused by bilateral shrunken kidneys. Other causes included hypertensive nephropathy and diabetic nephropathy, which were found in equal number in the study population. Hemodialysis was the mode of dialysis used by most of the patients (96%), while only two patients were on peritoneal dialysis (one in each group).
Overall, 25 patients (42.3%) received kidneys from deceased brain donors (DBDs), and 27 patients (57.7%) received kidneys from living donors. In group 1, 11 patients (42.3%) received organs from DBDs and 15 (57.7%) from a living donor. In group 2, 14 (53.9%) received kidneys from DBDs and 12 (46.1%) from living donors. With a living donor, the left kidney is preferred due to the long length of the renal vein relative to the right side, while cases of deceased donor transplants had an equal probability of receiving either kidney between groups. In the study population, the left kidney was used in 20 cases (76.9%) in group 1 and 17 cases (65.4%) in group 2. The right kidney was transplanted in six patients (23.1%) in group 1 and nine cases (34.6%) in group 2. Both groups were statistically comparable in terms of the side of kidney used for the renal transplant. The first transplant was usually performed in the right iliac fossa (RIF) except in special situations. RIF transplantation was conducted in 24 cases (92.3%) in group 1 and 22 cases (84.6%) in group 2.
The operative parameters are described in Table 3. The mean recipient vessel preparation time was calculated from identification of the external iliac vessels after reflecting the peritoneum and placing a self-retaining retractor to complete dissection of the external iliac vessels. A statistically significant difference was found between groups in the mean preparation time (8.3±1.9 minutes in group 1 and 14.5±4 minutes in group 2; P<0.001).
Two (7.7%) cases of donor artery in group 1 and four (15.4%) cases of donor artery in group 2 exhibited atherosclerosis, which was comparable between groups (P=0.385). Two cases of recipient artery in group 1 and three cases of recipient artery in group 2 had atherosclerosis (P=0.638).
Double renal arteries were encountered in three cases (11.5%) in each group. Side-to-side anastomosis of both renal arteries was done in all six cases. None of the patients in either group exhibited more than two vessels. The technique used for reconstruction of multiple vessels was comparable between groups. All cases had a single renal vein (P=1.000). The mean anastomosis time was almost equal between groups, at 28.2±5.4 minutes in group 1 and 28.2±4.2 minutes in group 2 (P=1.000). The mean estimated blood loss was 101.54±44.60 mL in group 1 and 125.19±74.17 mL in group 2 (P=0.270), representing a statistically insignificant difference.
An anticoagulant (dalteparin) was used in cases with risk of thrombosis. Six patients in group 1 and three in group 2 received anticoagulants for 10 days, followed by antiplatelets. An ancillary procedure (distal ureterectomy) was performed in one patient in group 1, who had reflux in the right native kidney. A drain was placed in seven patients in group 1 and 10 patients in group 2. A double-J stent was inserted in 16 patients in group 1 and 15 patients in group 2. Both groups were statistically comparable in these parameters (Table 4).
The postoperative serum creatinine level was measured in both groups on postoperative day 7. In group 1, the creatine level was 1.7±1.4 mg/dL, and in group 2, it was 1.2±2.4 mg/dL (P=0.728). Nadir serum creatinine was reached within 4.5 days in group 1 and within 4.1 days in group 2 (P=0.390). A drain was placed in seven of the 26 patients of group 1 and 10 of the 26 patients of group 2. The drain was removed on the day when output was <50 mL in 24 hours. Drain removal occurred comparatively early in group 1, with a mean drainage duration of 3.7 days compared to 4.3 days in group 2 (P=0.320). The mean drain output was lower in the EBV group, at 51.42 mL per day versus 57.50 mL per day in the ligature group (P=0.590).
Lymphocele was observed with equal incidence in both groups, with two cases found in each group. One in each group was <3 cm without obstructive changes and was asymptomatic; hence, these were followed up with using serial ultrasonography, as no intervention is required for lymphocele of <3 cm. One lymphocele in each group was >3 cm and lower polar in location. The lymphocele in group 1 presented with lymphocutaneous fistula following drain removal after a long duration due to high drain output, and open lymphocele deroofing was required during the same admission. The lymphocele in group 2 presented along with rising serum creatinine levels due to extrinsic compression by the lymphocele on the pelvis, so it was treated with laparoscopic lymphocele deroofing. The complications of both patients were relieved after the deroofing, and no recurrence was noted on follow-up. Exploration was required in two cases in group 2. One exhibited urinary leak, and one had postoperative hematoma. No exploration was done in group 1. Superficial and deep wound infections were comparable between groups. Vascular complication in the form of pseudoaneurysm of the renal artery was found in one patient in group 1 who underwent graft nephrectomy after 3 months posttransplant. Rates of graft rejection and non-lymphatic collection did not differ statistically between groups (Table 5). The mean hospital stay was 6.7±1.9 days in group 1 and 7.8±3.2 days in group 2 (P=0.136).
Following a renal transplant, lymphocele and lymphorrhoea are common lymphatic complications. Since the introduction of follow-up ultrasound imaging, the reported incidence of lymphocele has varied from 0.6% to 33.9%. It can occur 2 to 6 weeks posttransplant, with peak incidence at 6 weeks. The mean reported incidence of symptomatic lymphocele is only 5.2%. Lymphocele <3 cm in diameter usually resolves spontaneously, and most of the lymphatic collections are subclinical. The development of lymphocele can be based on surgical and medical factors. Two possible sources of lymphorrhoea are recipient lymphatics and graft lymphatics [7]. The lymphocele originates from the leakage of unligated lymphatics. Thus, careful ligation of all lymphatics in the vicinity of iliac vessels is recommended.
In the present prospective randomized clinical study, outcomes were compared between EBVS LigaSure (Medtronic) and conventional ligation with sutures for the control of perivascular lymphatic channels in open renal transplant recipients. The results suggested that the mean anastomotic time, estimated blood loss, day of drain removal, mean drain output per day, time to reach nadir creatinine levels, and mean hospital stay were statistically similar between groups. Based on the present study, using of the LigaSure (Medtronic) device is less time-consuming while being safe and effective for the control of recipient iliac lymphatic vessel dissection and sealing. The mean time for lymphatic sealing and preparation of the recipient vessels was significantly different between groups, at 8.3 minutes with EBVS versus 14.5 minutes with silk suture ligation. The rate of posttransplant lymphatic complications, such as lymphorrhoea and lymphocele, also appears to be minimal. However, relative to conventional ligation, notwithstanding these advantages of LigaSure (Medtronic), cost-effectiveness may be a constraint in developing countries like India.
Hamza et al. [8] recommended that to prevent lymphocele formation, transplant recipient vessel dissection be minimized and lymphatic vessels be ligated precisely at the hilum of the kidney allograft. Ligation of lymphatic vessels during preparation of either the graft or the transplantation site, along with appropriate external drainage, can reduce the incidence of lymphocele. The incidence of other postoperative fluid collections such as urinoma, seroma, hematoma, or abscess has decreased markedly due to improved surgical techniques and skills [9].
No single surgical technique has proven superior to others for preventing lymphocele. Some studies have indicated the efficacy of various surgical methods, while others have found no statistically significant differences. Alternatives to conventional ligature for the control of lymphatics include monopolar and bipolar electrocautery, ultrasonic thermal sealing, and feedback-controlled electrothermal bipolar energy sources such as the LigaSure sealing device (Valleylab, Boulder, CO, USA) and plasma kinetic sealer (Gyrus Medical Inc., Maple Grove, MN, USA) [5].
The LigaSure (Medtronic) technology is a type of EBVS device that fuses vessels using a combination of pressure and energy. This denatures the collagen, elastin, and other connective tissue within the vessel and allows the proteins to form a seal, which fuses with the wall. In this manner, the lumen is theoretically obliterated, and little blood or lymph leak occurs [9]. The first report to establish the efficacy of EBVS for the sealing of lymphatic vessels in an animal model was published by Novitsky et al. [5] in 2005. In a study conducted on pigs, 15 seals were analyzed for sealing time, visual quality, and seal burst strength. The researchers concluded that EBVS yields fast and effective sealing of large porcine lymphatic vessels [5].
In a meta-analysis of 29 prospective randomized trials, LigaSure (Medtronic) was compared with suture ligation/electrocauterization or the harmonic scalpel in various surgical procedures, including hemorrhoidectomy (12 articles), hysterectomy (four articles) and thyroidectomy (three articles). With EBVS, reductions were observed in operative time (P<0.001), blood loss (P=0.002), postoperative pain (P<0.001) and complications (P=0.020) [10]. Recently, the use of the LigaSure (Medtronic) EBVS has proven superior to other vessel sealing techniques in several reports, many of them in breast surgery, both for lymphatic and blood vessel sealing. In a study by Panhofer et al. [11], the use of LigaSure (Medtronic) halved the incidence of seroma and shortened the duration of hospital stay by an average of 1 day in a cohort of female patients undergoing either breast-conserving surgery or isolated axillary lymph node dissection. Tsuda et al. [12], however, reported no statistically significant difference in the frequency of seroma when comparing LigaSure (Medtronic) with conventional dissection techniques.
Nespoli et al. [13], reported only a marginal advantage of LigaSure (Medtronic) use over conventional methods, while another study by Panhofer et al. [11], found no statistically significant difference between the two methods in axillary lymph node dissection surgery. Studies in pelvic surgery have demonstrated the superiority of LigaSure (Medtronic) over other methods, such as in a study by Tsuda et al. [12], where a notable difference in the incidence of symptomatic lymphocele was found with EBVS relative to tie ligation (5.3% vs. 14%, P<0.001) during pelvic lymphadenectomy in gynaecological cancers. Regarding kidney transplant, a retrospective study on the use of LigaSure (Medtronic) for arterial and venous sealing in living-donor nephrectomy has yielded results in favour of this method [14]. Most studies of the LigaSure (Medtronic) device have shown advantages compared to other methods in terms of sealing time, burst pressure, thermal spread, intraoperative blood loss, operative time, conversion rate, and postoperative course.
In a retrospective study conducted by Mok et al. [14] in 2021, the records of 100 kidney transplantation patients were analyzed. Two groups comprised 50 patients each. In one group, LigaSure (Medtronic) was used as a method to seal the lymphatics, while the conventional lymphatic ligation technique was utilised in the other. Unlike the present study, this was a retrospective analysis, and the groups were also confounded by intergroup differences in hypertension history, number of renal arteries anastomosed, and the anastomosis techniques used. The researchers performed a subgroup analysis depending on the pattern of anastomosis. They concluded that no significant difference was present in drain removal time or lymphocele incidence. However, in the subgroup analysis, greater drain volume was observed at postoperative day 1 in patients with end-to-end internal iliac artery-renal artery anastomosis. According to the study, this may be because the internal iliac artery preparation requires more dissection with longer lymphatic ligation.
In another randomized trial by Simforoosh et al. [15] comparing electrothermal bipolar cautery with suture ligature in the prevention of lymphocele formation after renal transplant, 60 patients were studied. The researchers utilised ultrasonography at 5 months post-surgery to analyze the status of lymphocele formation. In this study, 25 patients underwent living-donor kidney transplantation, and 35 underwent deceased-donor kidney transplantation. The time for suture ligation or bipolar cauterization of lymphatic vessels was similar between the two groups, ranging from 9 to 23 minutes overall with the same average (15.73 minutes) in both groups. No lymphocele collection, either symptomatic or asymptomatic, was seen on ultrasonography at 5-month follow-up. No significant difference in postoperative pain was observed between the two groups. The mean postoperative drainage duration was 5.6 days in the suture ligature group compared to 6.07 days in the bipolar cautery group, which did not constitute a significant difference.
This study had some limitations. The length of external iliac vessels dissected, and hence the amount of lymphatic dissection, varied among surgeons doing the recipient vessel preparation and also varied according to patient characteristics. For example, in an obese patient, working at depth requires better exposure, with a long dissection of vessels to make a vascular anastomosis comfortable. This could present a confounding effect on the results.
The present prospective comparative study reveals that EBVS can be employed safely and effectively for recipient iliac lymphatic vessel dissection and sealing. EBVS has certain advantages relative to conventional ligation. Control of lymphocele by EBVS is significantly quicker than silk ligation, and postoperative lymphocele and lymphorrhoea are comparable to silk suture ligature. However, to further validate and recommend the use of the EBVS device as the technique of choice in all lymphatic dissection and sealing during renal transplantation, larger comparative studies are required. Moreover, cost-effectiveness is a major constraint among the populations of developing countries.
REFERENCES
1. Abecassis M, Bartlett ST, Collins AJ, Davis CL, Delmonico FL, Friedewald JJ, et al. 2008; Kidney transplantation as primary therapy for end-stage renal disease: a National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin J Am Soc Nephrol. 3:471–80. DOI: 10.2215/CJN.05021107. PMID: 18256371. PMCID: PMC2390948.
2. Campi R, Pecoraro A, Li Marzi V, Tuccio A, Giancane S, Peris A, et al. 2022; Robotic versus open kidney transplantation from deceased donors: a prospective observational study. Eur Urol Open Sci. 39:36–46. DOI: 10.1016/j.euros.2022.03.007. PMID: 35528789. PMCID: PMC9068739.
3. Furness PD 3rd, Houston JB, Grampsas SA, Karrer FM, Firlit CF, Koyle MA. 2001; Extraperitoneal placement of renal allografts in children weighing less than 15 kg. J Urol. 166:1042–5. DOI: 10.1016/S0022-5347(05)65915-0. PMID: 11490294.
4. Ebadzadeh MR, Tavakkoli M. 2008; Lymphocele after kidney transplantation: where are we standing now? Urol J. 5:144–8. PMID: 18825619.
5. Novitsky YW, Rosen MJ, Harrell AG, Sing RF, Kercher KW, Heniford BT. 2005; Evaluation of the efficacy of the electrosurgical bipolar vessel sealer (LigaSure) devices in sealing lymphatic vessels. Surg Innov. 12:155–60. DOI: 10.1177/155335060501200215. PMID: 16034506.
6. Khan TF, Baig MA. 2011; Post kidney transplant lymphoceles: meticulous ligation of lymphatics reduces incidence. Urotoday Int J. 4:art 64. DOI: 10.3834/uij.1944-5784.2011.10.07.
7. Lucan CV, Jurchis I, Suciu M, Selicean SE, Buttice S. 2017; Modern lymphatic dissection techniques for preventing post renal transplant lymphocele. Clujul Med. 90:416–9. DOI: 10.15386/cjmed-716. PMID: 29151791. PMCID: PMC5683832.
8. Hamza A, Fischer K, Koch E, Wicht A, Zacharias M, Loertzer H, et al. 2006; Diagnostics and therapy of lymphoceles after kidney transplantation. Transplant Proc. 38:701–6. DOI: 10.1016/j.transproceed.2006.01.065. PMID: 16647449.
9. Heniford BT, Matthews BD, Sing RF, Backus C, Pratt B, Greene FL. 2001; Initial results with an electrothermal bipolar vessel sealer. Surg Endosc. 15:799–801. DOI: 10.1007/s004640080025. PMID: 11443443.
10. Kennedy JS, Stranahan PL, Taylor KD, Chandler JG. 1998; High-burst-strength, feedback-controlled bipolar vessel sealing. Surg Endosc. 12:876–8. DOI: 10.1007/s004649900733. PMID: 9602010.
11. Panhofer P, Rothe S, Schütz M, Grohmann-Izay B, Dubsky P, Jakesz R, et al. 2015; Morbidity reduction using the vessel sealing device LigaSure™ in breast cancer surgery. Eur Surg. 47:150–6. DOI: 10.1007/s10353-015-0334-8.
12. Tsuda N, Ushijima K, Kawano K, Takemoto S, Nishio S, Sonoda G, et al. 2014; Prevention of lymphocele development in gynecologic cancers by the electrothermal bipolar vessel sealing device. J Gynecol Oncol. 25:229–35. DOI: 10.3802/jgo.2014.25.3.229. PMID: 25045436. PMCID: PMC4102742.
13. Nespoli L, Antolini L, Stucchi C, Nespoli A, Valsecchi MG, Gianotti L. 2012; Axillary lymphadenectomy for breast cancer. A randomized controlled trial comparing a bipolar vessel sealing system to the conventional technique. Breast. 21:739–45. DOI: 10.1016/j.breast.2012.08.003. PMID: 22959311.
14. Mok S, Park YJ, Park SC, Yun SS. 2021; Efficacy of lymphatic sealing using the LigaSure in kidney transplantation: a pilot study. Transplant Proc. 53:2278–84. DOI: 10.1016/j.transproceed.2021.07.020. PMID: 34404537.
15. Simforoosh N, Tabibi A, Rad HM, Gholamrezaie HR. 2019; Comparison between bipolar lymphatic vessels cautery and suture ligature in prevention of postrenal transplant lymphocele formation: a randomized controlled trial. Exp Clin Transplant. 17:26–30. DOI: 10.6002/ect.2017.0207. PMID: 29697354.
Fig. 1
Lymphatics sealed with LigaSure (Medtronic). (A) Lymphatics (arrow) over the external iliac vessels (asterisk) dissected with Mixter (Sklar) right-angle forceps. (B) Dissected lymphatic controlled with a small-jaw curved LigaSure (arrow). (C) Completely dissected external iliac artery (arrow) and vein (asterisk).
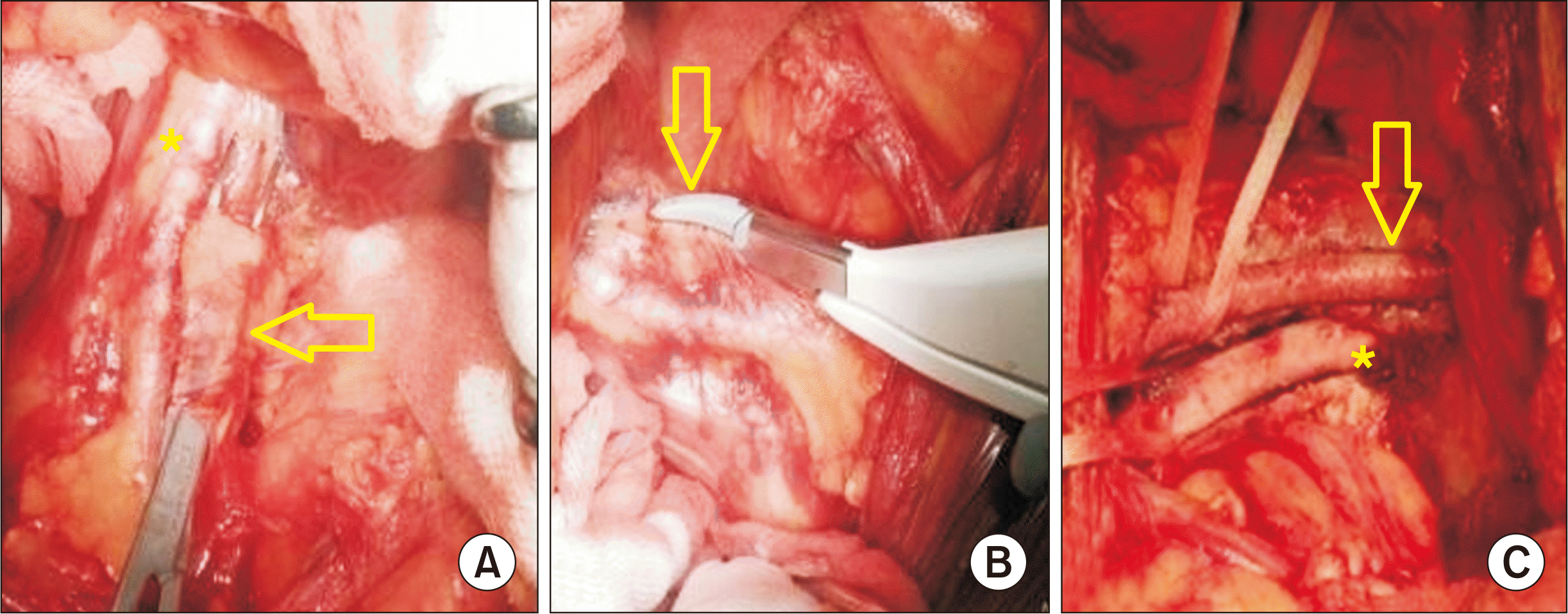
Table 1
Characteristics of recipients and donors
Table 2
Causes of ESRD
Table 3
Characteristics related to surgery
Table 4
Other intraoperative variables
Table 5
Postoperative non-lymphatic complications




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download