Abstract
Background
During transplantation, a kidney graft undergoes a cascade of pathological changes, referred to as ischemia-reperfusion injury (IRI), as it is incorporated into the bloodstream. Various studies have reported that retrograde reperfusion (RRP) leads to improved myocardial recovery and could reduce IRI in liver transplantation. This study investigated the effect of RRP in renal transplantation with a focus on reduction of kidney IRI.
Methods
Between December 2019 and July 2022, 15 consecutive kidney transplants were performed with retrograde venous reperfusion. To conduct a comparative study and to recruit a control group, 15 kidney transplants that had been performed in the same center by the same two surgeons were retrospectively analyzed. Differences between the two groups were considered statistically significant at P<0.05.
Results
The baseline characteristics of the two groups were statistically comparable (P>0.05). The surgical technique for kidney transplantation was the same in both groups. On the first postoperative day, polyuria was less pronounced in the RRP group (P<0.01). Serum creatinine and urea levels and estimated glomerular filtration rates on postoperative days 1, 4, 7, and 30 were lower in the RRP group (P<0.05).
Organ transplantation has proven its effectiveness in the treatment of end-stage organ and system diseases, as well as its economic benefits [1,2]. Despite advances in transplantology and immunology, the 1-year survival rate of kidney transplants is 92%–95% for transplants from a living donor and 89%–90% for transplants from a cadaveric donor [3,4]. Approximately half of kidney transplant losses (47%) are due to rejection and the secondary effects of immunosuppressive drugs (i.e., negative immunologically mediated causes) [5]. In addition, a kidney graft undergoes a cascade of pathological changes referred to as an ischemia-reperfusion injury (IRI) when it is incorporated into the bloodstream. IRI is an unavoidable part of kidney transplantation surgery. Ischemia and reperfusion initiate a cascade of immuno-inflammatory reactions that directly affect the function of the graft and, in severe cases, can lead to complete loss of the graft. Therefore, approximately 20% of recipients have delayed graft function, and this is more common in cadaveric transplants than in live-donor transplants (29% vs. 17%) [6]. Oxygen free radicals are the primary detrimental factors in IRI [7], causing a pronounced inflammation of the endothelium that can lead to occlusion of small vessels and a decrease in perfusion called the reperfusion paradox. In simplified terms, a high flow of blood leads to hypoperfusion of the renal graft due to the detrimental effects of oxygen free radicals [8].
Various studies have explored whether reversed reperfusion might lead to a diminished production of oxygen free radicals. Carpenter et al. [9] found that retrograde reperfusion (RRP) with low-oxygenated blood after cardiac bypass surgery improved the myocardial recovery time and decreased myocardial cellular injury. In addition, RRP via the vena cava prior to anterograde reperfusion of the hepatic artery and portal vein also led to a reduction of IRI in liver transplantation. Slow elution of perfusion solution, slow rewarming, and slow re‐oxygenation with low‐oxygenated blood might reduce the production of oxygen free radicals. An Austrian study investigated this new reperfusion technique in a retrospective study of 42 consecutive liver transplants and showed that, in contrast to reports in the literature, circulatory problems or electrolyte imbalances after declamping of the anastomosis were very uncommon in their series [10]. Another study demonstrated that RRP in combination with portal arterial liver transplantation showed excellent clinical effects compared to other methods of reperfusion [11]. Another experimental investigation proved that retrograde oxygen persufflation was able to protect and restore kidney parenchyma [12]. Daniela et al. [13] found that postreperfusion syndrome occurred in only 3.6% of study subjects following RRP compared with the proportion of approximately 20% reported in the literature. They reported no initial nonfunction of liver grafts in their series.
This study was approved by the Institutional Review Board of West-Kazakhstan Medical University (IRB No. 55A.02.09.2021) and registered with ClinicalTrials.gov (identifier: NCT05179434). Written informed consent was obtained from all participants.
Between December 2019 and July 2022, 15 consecutive kidney transplants were performed using retrograde venous reperfusion of the renal graft. To conduct a comparative study and to recruit a control group, 15 kidney transplants that had been performed at the same center by the same two surgeons were retrospectively analyzed.
The trial was monocentric and retrospectively controlled. The criteria for inclusion of patients with chronic kidney disease in the two groups were: adults, primary kidney transplantation (no retransplantation), no history of malignancy, panel reactive antibodies <20% (to exclude immunological effects on the kidney transplant), and transplantation of only one solid organ (kidney) (Fig. 1). In both groups, patients had the same three-component immunosuppressive regime: calcineurin inhibitors (tacrolimus), mofetil mycophenolate, and a steroid. The recruited patients were divided into two groups: the main group with RRP (n=15) and the control group without RRP (n=15).
All 30 patients underwent classic kidney transplantation from a living donor. The detailed surgical technique of retrograde venous reperfusion was as follows: after conventional venous anastomosis, the renal and iliac arteries were anastomosed without tightening the suture to leave a lumen sufficient for the outflow of retrograde blood [16,17]. The recipient’s iliac artery remained completely clamped; Next, the retrograde blood flow through the renal vein was started. Venous blood (80–100 mL) filled the graft and flowed through the renal artery and through the lumen of the anastomosis (Fig. 2) [18]; next, the sutures of the arterial anastomosis were tightened. After tying, the iliac artery was unclamped and a typical antegrade reperfusion of the graft through the renal artery was achieved [19,20].
In the main group with RRP, retrograde blood was taken from the renal artery for analysis using a blood gas analyzer. In both groups, the recipients’ diuresis on the first postoperative day, as well as their serum urea levels, serum creatinine levels, and estimated glomerular filtration rate (eGFR) (using the Modification of Diet in Renal Disease study equation) on postoperative days 1, 4, 7, 14, and 30 were compared.
The baseline clinical characteristics of both groups are shown in Table 1. There were no significant differences between the two groups in age, body mass index (BMI), duration of preoperative dialysis, initial urea and creatinine levels, and eGFR before transplantation. In both groups, the recipients’ BMI did not exceed 29 kg/m2, and there were equal numbers of men and women. There were also no differences in intraoperative characteristics: duration of surgery (the time from the beginning of the first incision to the last suture of the wound; P>0.05), cold ischemic time (the time from when perfusion of the graft started to the start of venous anastomosis; P>0.05), and warm ischemic time (the time from when vein anastomosis started to arterial reperfusion of the renal graft; P>0.05).
In all cases of kidney transplantation with RRP, the graft function was satisfactory. Complications such as vascular thrombosis were not observed, and during the operation there were no vascular reanastomosis. In the comparative analysis of retrograde blood gases to blood tests taken directly from the iliac vein, the pH, base excess of extracellular fluid, and calcium levels were lower (P<0.01), and the lactate and potassium levels were higher (P<0.01) in Table 2.
The postoperative characteristics of the two groups are presented in Table 3. On the first postoperative day, polyuria was less pronounced in the main group (P<0.01). Serum creatinine levels, urea levels, and the eGFR on postoperative days 1, 4, 7, and 30 were lower in the main group (P<0.05). However, these levels did not differ on postoperative day 14.
IRI in transplanted organs is a key factor affecting the subsequent function and survival of the graft [20]. To reduce the negative effects of ischemia and renal reperfusion during the transplantation process, various methods have been proposed including dietary preconditioning, various organ storage methods, various types of machine perfusion, ischemic preconditioning and postconditioning, the use of mesenchymal stem cells, and treatment with pharmacological preparations and microRNAs [21].
RRP has mainly been used in cardiac surgery and liver transplantation, and we did not find studies in the available literature on using this technique in kidney transplantation. Nevertheless, the immuno-inflammatory processes occurring after ischemia and reperfusion are similar for both liver cells and kidney tissue. In our study, analysis of the acid-base balance and gases of the retrograde blood showed that the renal tissue was in a hypometabolic state with a shift in acidity toward deep acidosis. At the same time, by releasing 80–100 mL of retrograde blood, harmful metabolic products were removed without entering the general bloodstream, a result identical to that described by other researchers investigating RRP during liver transplantation [18]. Some studies have shown that initial perfusion of organs with venous blood at low pressure and low oxygen concentration, with a gradual increase in pressure and oxygenation and gradual rewarming of the organ, can reduce the production of free oxygen radicals and IRI [18,22,23].
After acute injury of the renal parenchyma in the early period of the recovery stage, polyuria of varying severity has been noted. After ischemia and the beginning of organ reperfusion, tubular regeneration begins, but the functional maturation of the tubules may slow down with a decrease in the reabsorption ability of the kidney [24]. In this study, we found no pronounced polyuria in the main group compared to the control group. Apparently, increased polyuria may be associated with the depth of the IRI in kidney tissue.
In the early postoperative period, the serum creatinine and urea levels significantly decreased in the group with RRP, and returned to normal on day 4 after the transplantation. The eGFR was also higher and the creatinine and urea levels were lower in the RRP group than in the control group over the long term (postoperative day 30). It is possible that RRP of the renal grafts with venous blood prevented massive outbreaks of oxygen free radicals and, thereby, reduced the degree of IRI.
A histomorphological examination of the kidneys would have more clearly demonstrated possible differences between the two groups. However, none of the recipients in the main group had indications for a biopsy during the observation period. Retrograde venous reperfusion of kidney transplants, preceding antegrade arterial reperfusion, reduced the effects of renal parenchyma IRI. To validate these results, it is necessary to conduct further studies on a larger cohort of patients with a longer follow-up period.
ACKNOWLEDGMENTS
Funding/Support
The project was awarded "The International Research Grant" during the Asian Transplantation Week (ATW) 2020 by the Korean Society for Transplantation in December 2020.
REFERENCES
1. Axelrod DA, Schnitzler MA, Xiao H, Irish W, Tuttle-Newhall E, Chang SH, et al. 2018; An economic assessment of contemporary kidney transplant practice. Am J Transplant. 18:1168–76. DOI: 10.1111/ajt.14702. PMID: 29451350.
2. Asgeirsdóttir TL, Asmundsdóttir G, Heimisdóttir M, Jónsson E, Pálsson R. 2009; Cost-effectiveness analysis of treatment for end-stage renal disease. Laeknabladid. 95:747–53. PMID: 19996463.
3. Hart A, Lentine KL, Smith JM, Miller JM, Skeans MA, Prentice M, et al. 2021; OPTN/SRTR 2019 annual data report: kidney. Am J Transplant. 21 Suppl 2:21–137. DOI: 10.1111/ajt.16502. PMID: 33595191.
4. Lentine KL, Smith JM, Hart A, Miller J, Skeans MA, Larkin L, et al. 2022; OPTN/SRTR 2020 annual data report: kidney. Am J Transplant. 22 Suppl 2:21–136. DOI: 10.1111/ajt.16982. PMID: 35266618.
5. Lohéac C, Aubert O, Loupy A, Legendre C. 2018; Identifying the specific causes of kidney allograft loss: a population-based study. Nephrol Ther. 14 Suppl 1:S39–50. DOI: 10.1016/j.nephro.2018.02.018. PMID: 29606262.
6. Han F, Lin MZ, Zhou HL, Li H, Sun QP, Huang ZY, et al. 2020; Delayed graft function is correlated with graft loss in recipients of expanded-criteria rather than standard-criteria donor kidneys: a retrospective, multicenter, observation cohort study. Chin Med J (Engl). 133:561–70. DOI: 10.1097/CM9.0000000000000666. PMID: 32053570. PMCID: PMC7065861.
7. Minor T, Isselhard W, Klauke H. 1996; Reduction in nonparenchymal cell injury and vascular endothelial dysfunction after cold preservation of the liver by gaseous oxygen. Transpl Int. 9 Suppl 1:S425–8. DOI: 10.1111/j.1432-2277.1996.tb01667.x. PMID: 8959878.
8. Menger MD, Richter S, Yamauchi J, Vollmar B. 1999; Role of microcirculation in hepatic ischemia/reperfusion injury. Hepatogastroenterology. 46 Suppl 2:1452–7. PMID: 10431706.
9. Carpenter AJ, Follette DM, Sheppard B, Yoshikawa R, Sam J. 1999; Simultaneous antegrade and retrograde reperfusion after cardioplegic arrest for coronary artery bypass. J Card Surg. 14:354–8. DOI: 10.1111/j.1540-8191.1999.tb01008.x. PMID: 10875589.
10. Kniepeiss D, Iberer F, Grasser B, Schaffellner S, Stadlbauer V, Tscheliessnigg KH. 2003; A single-center experience with retrograde reperfusion in liver transplantation. Transpl Int. 16:730–5. DOI: 10.1111/j.1432-2277.2003.tb00232.x. PMID: 12819865.
11. Yao Y, Wu P, Guo T. 2019; Identifying the superior reperfusion technique in liver transplantation: a network meta-analysis. Gastroenterol Res Pract. 2019:9034263. DOI: 10.1155/2019/9034263. PMID: 31641349. PMCID: PMC6766671.
12. Molacek J, Opatrný V, Treska V, Matejka R, Hes O. 2018; Options to improve the quality of kidney grafts from expanded criteria donors experimental study. Rozhl Chir. 97:193–201. PMID: 29792716.
13. Daniela K, Michael Z, Florian I, Silvia S, Estrella J, Doris D, et al. 2004; Influence of retrograde flushing via the caval vein on the post-reperfusion syndrome in liver transplantation. Clin Transplant. 18:638–41. DOI: 10.1111/j.1399-0012.2004.00231.x. PMID: 15516236.
14. Srinivasan PK, Yagi S, Nagai K, Afify M, Hata K, Uemoto S, et al. 2014; Impact of oxygen free radicals in rat partial liver transplantation. J Surg Res. 191:469–75. DOI: 10.1016/j.jss.2014.05.002. PMID: 24888787.
15. Maring JK, Klompmaker IJ, Zwaveling JH, van Der Meer J, Limburg PC, Slooff MJ. 2000; Endotoxins and cytokines during liver transplantation: changes in plasma levels and effects on clinical outcome. Liver Transpl. 6:480–8. DOI: 10.1053/jlts.2000.8311. PMID: 10915172.
16. Duran JA, González AA, García DD, Falcón RC, Pereda PS, Navarro J, et al. 2009; Best blood sample draw site during liver transplantation. Transplant Proc. 41:991–3. DOI: 10.1016/j.transproceed.2009.02.034. PMID: 19376406.
17. Chung HS, Lee HS, Lee J, Choi JH, Park CS. P183 serum cytokines as predictors of liver graft dysfunction based on the model for end-stage liver disease score in patients undergoing living donor liver transplantation. Hum Immunol. 2017; 78 Suppl:194. DOI: 10.1016/j.humimm.2017.06.243.
18. Yang C, Huang L, Li X, Zhu J, Leng X. 2018; Effects of retrograde reperfusion on the intraoperative internal environment and hemodynamics in classic orthotopic liver transplantation. BMC Surg. 18:115. DOI: 10.1186/s12893-018-0441-0. PMID: 30541532. PMCID: PMC6292078.
19. Kern H, Bald C, Brill T, Fend F, von Weihern CH, Kriner M, et al. 2008; The influence of retrograde reperfusion on the ischaemia-/reperfusion injury after liver transplantation in the rat. Int J Exp Pathol. 89:433–7. DOI: 10.1111/j.1365-2613.2008.00616.x. PMID: 19134052. PMCID: PMC2669604.
20. Salvadori M, Rosso G, Bertoni E. 2015; Update on ischemia-reperfusion injury in kidney transplantation: pathogenesis and treatment. World J Transplant. 5:52–67. DOI: 10.5500/wjt.v5.i2.52. PMID: 26131407. PMCID: PMC4478600.
21. Saat TC, van den Akker EK, IJzermans JN, Dor FJ, de Bruin RW. 2016; Improving the outcome of kidney transplantation by ameliorating renal ischemia reperfusion injury: lost in translation? J Transl Med. 14:20. DOI: 10.1186/s12967-016-0767-2. PMID: 26791565. PMCID: PMC4721068.
22. Mahboub P, Ottens P, Seelen M, 't Hart N, Van Goor H, Ploeg R, et al. 2015; Gradual rewarming with gradual increase in pressure during machine perfusion after cold static preservation reduces kidney ischemia reperfusion injury. PLoS One. 10:e0143859. DOI: 10.1371/journal.pone.0143859. PMID: 26630031. PMCID: PMC4667888.
23. Tasoulis MK, Douzinas EE. 2016; Hypoxemic reperfusion of ischemic states: an alternative approach for the attenuation of oxidative stress mediated reperfusion injury. J Biomed Sci. 23:7. DOI: 10.1186/s12929-016-0220-0. PMID: 26786360. PMCID: PMC4717563.
24. Ramoutar V, Landa C, James LR. 2014; Acute tubular necrosis (ATN) presenting with an unusually prolonged period of marked polyuria heralded by an abrupt oliguric phase. BMJ Case Rep. 2014:bcr2013201030. DOI: 10.1136/bcr-2013-201030. PMID: 25150229. PMCID: PMC4154042.
Fig. 1
Study design to assess retrograde reperfusion (RRP) of renal grafting. PRA, panel reactive antibodies.
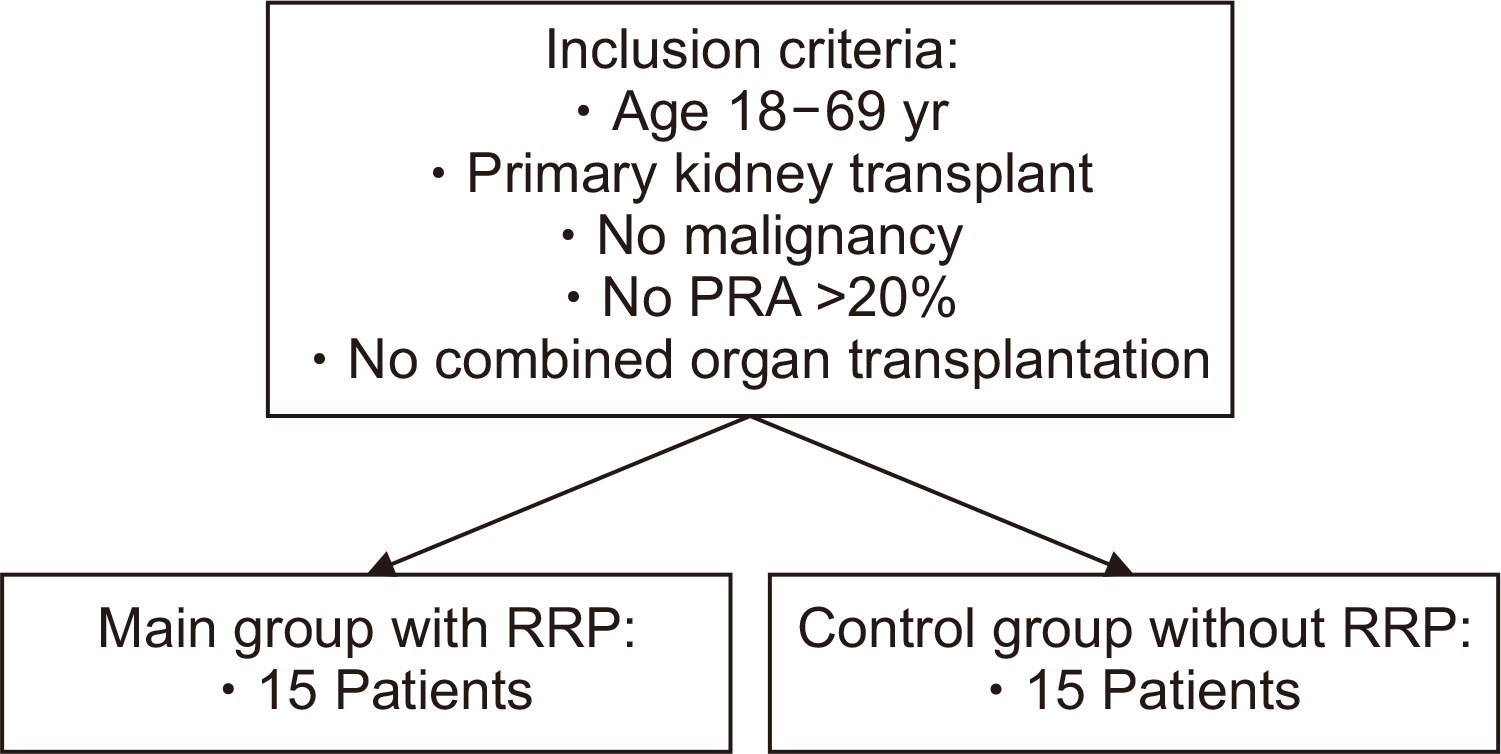
Fig. 2
Retrograde reperfusion of renal graft. (A) Retrograde reperfusion through renal vein; iliac artery is clamped. (B) Retrograde blood collection from the arterial anastomosis lumen.
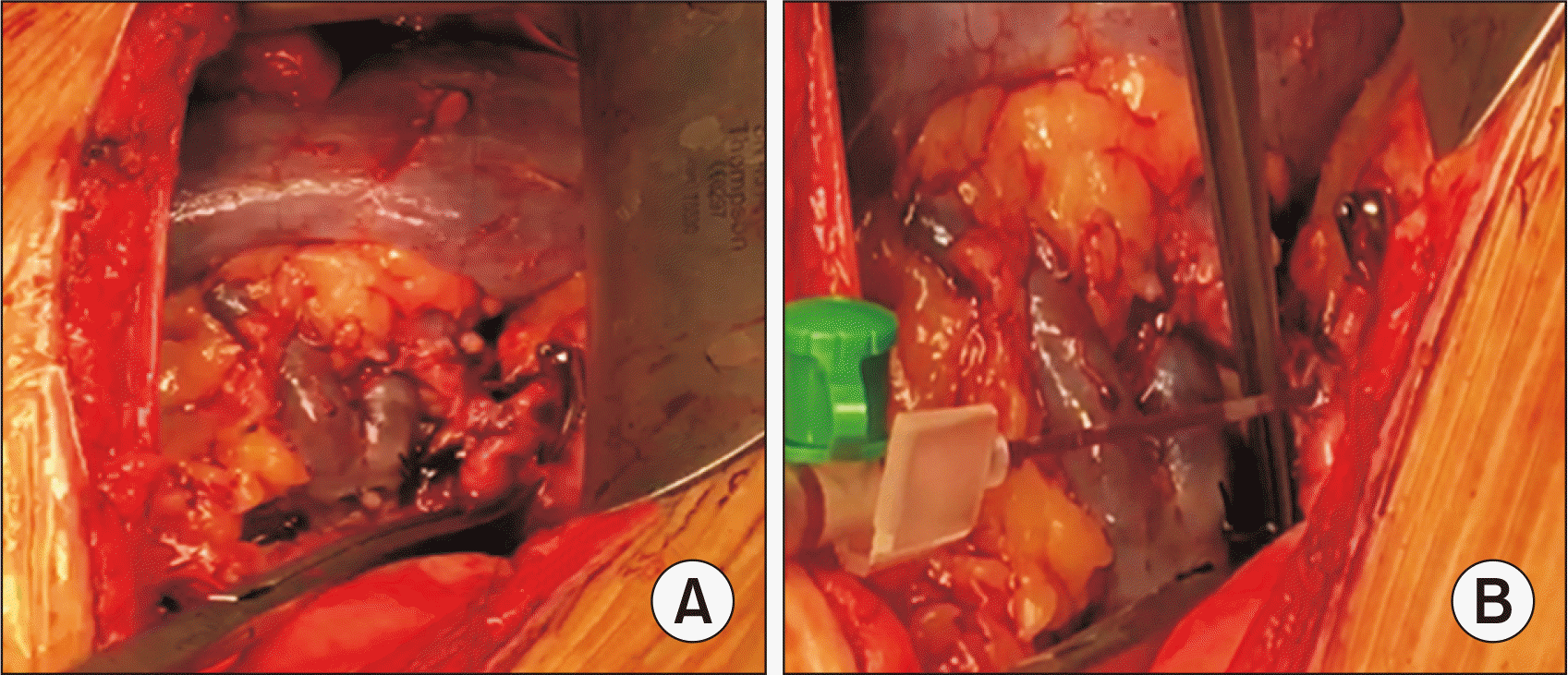
Table 1
Comparison of the baseline characteristics of the two renal transplant recipient groups
Table 2
Retrograde and iliac vein blood gas analyses
Table 3
Comparative postoperative data of the two renal transplant groups




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download