Abstract
Metastatic pulmonary calcification (MPC) is defined as calcium deposition in lung tissues. It is commonly seen in end-stage renal disease patients. However, MPC occurring in kidney transplant recipients (KTRs) is rare. We report a case of MPC in a 55-year-old female patient after successful kidney transplantation (KT). One year after KT, bisphosphonate and vitamin D were prescribed for osteoporosis. Then, 4.5 years after KT, we incidentally found multiple nodular lesions on chest X-ray (CXR) without any symptoms. Chest computed tomography showed multiple high-density nodules. A bone scan confirmed MPC in the right middle lobe and right lower lobe. A retrospective review of pretransplant blood chemistry revealed the following: serum calcium level, 11.2 mg/dL; phosphorus level, 3.2 mg/dL; intact parathyroid hormone level, lower than 2.5 pg/mL; and 24-hour urine calcium level, within normal limits (WNL). After KT, all of these parameters remained WNL. Therefore, hidden adynamic bone disease might have been aggravated by bisphosphonate and vitamin D supplementation, causing MPC. Both were discontinued. She was monitored by routine CXR, and MPC did not progress. Since MPC is commonly asymptomatic and difficult to diagnose in KTRs, caution is required when administering such medications. Patient should be followed up with routine CXR.
Metastatic pulmonary calcification (MPC) is defined as calcium deposition in lung tissues without prior tissue damage. It is commonly seen in end-stage renal disease (ESRD) patients on dialysis [1,2]. Although the mechanism underlying the occurrence of MPC has not been clearly identified, MPC in dialysis patients is associated with secondary hyperparathyroidism, which increases calcium and phosphate release from bone, while intermittent alkalosis during hemodialysis provides a background that is conducive for calcium salt precipitation in lung soft tissues [3]. Decreased glomerular filtration of phosphate contributes to the elevation of the calcium-phosphate product [4].
MPC is rarely found in individuals with normal renal function whose serum calcium and phosphate levels are within normal ranges [5]. After ESRD patients receive kidney transplantation (KT), most risk factors for MPC, such as serum calcium-phosphate imbalance, secondary hyperparathyroidism, and metabolic acidosis or alkalosis, are corrected [6]. Thus, new-onset MPC after KT is rarely seen. However, MPC can occur after successful KT in exceptional cases. Here we report a rare case of MPC in a patient after KT.
This study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital (IRB No. KC22ZASI0311). Informed consent was waived due to the retrospective nature of this study.
A 55-year-old female patient diagnosed with ESRD of unknown origin was referred to Seoul St. Mary’s Hospital for pre-emptive KT. She was never on dialysis. The donor was her 24-year-old son. The human leukocyte antigen (HLA) mismatch number was 3. Panel reactive antibody and crossmatch results were all negative. No specific abnormal findings were noted on the pretransplant chest images. With basiliximab induction, KT was performed successfully. She was discharged 2 weeks after KT with a serum creatinine level of 0.61 mg/dL. Triple immunosuppressants (a steroid, tacrolimus, and mycophenolic acid) were maintained.
One year after KT, the patient was diagnosed with osteoporosis in routine follow-up of bone mineral density, with a T-score of –3.1. Bisphosphonate and cholecalciferol (vitamin D) were prescribed. Since then, she presented to our outpatient clinic for follow-up at least every 3 months. Her blood chemistry results, including serum creatinine, calcium, phosphorus, and intact parathyroid hormone (iPTH), remained within their normal limits (WNL).
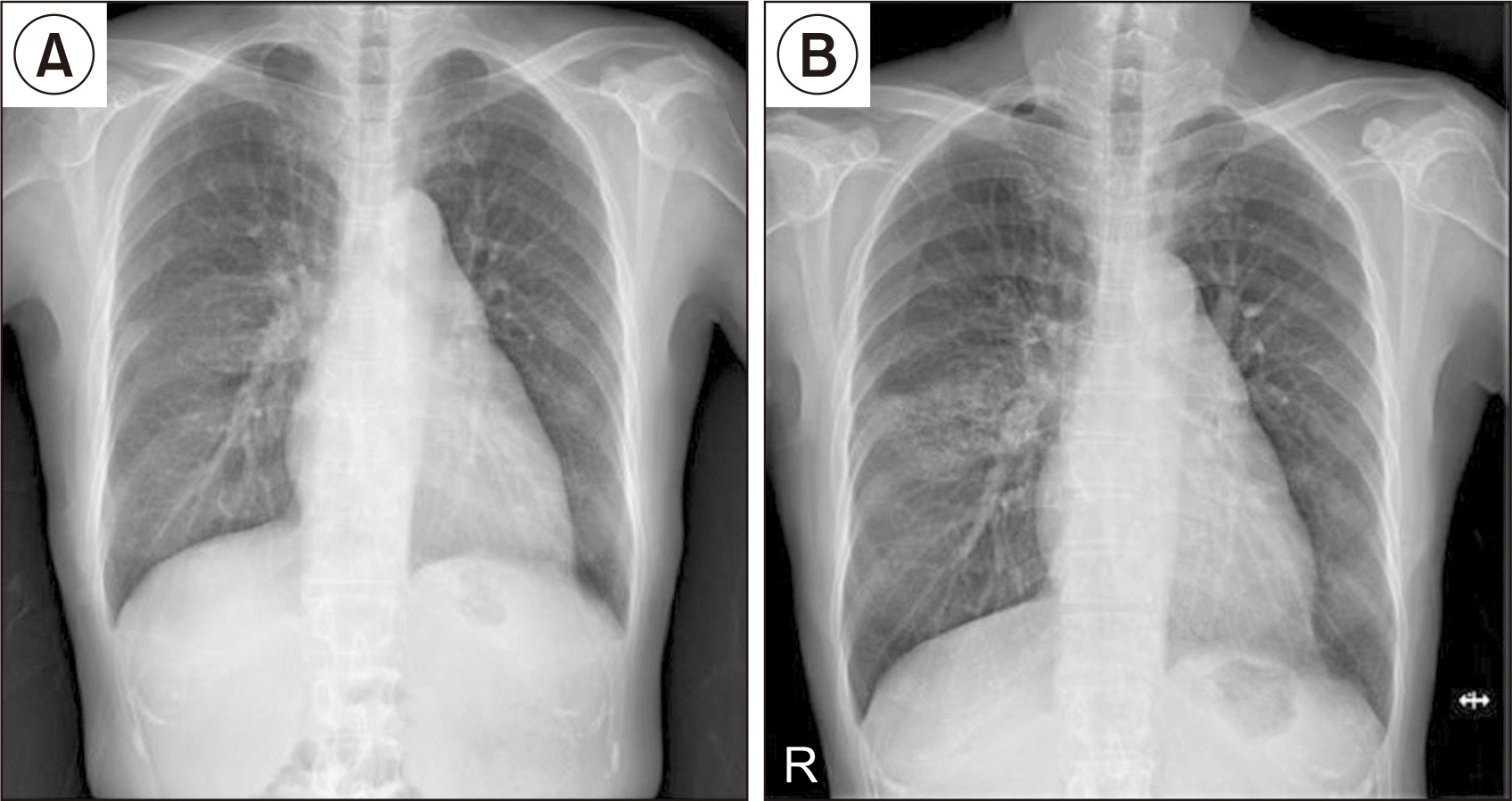
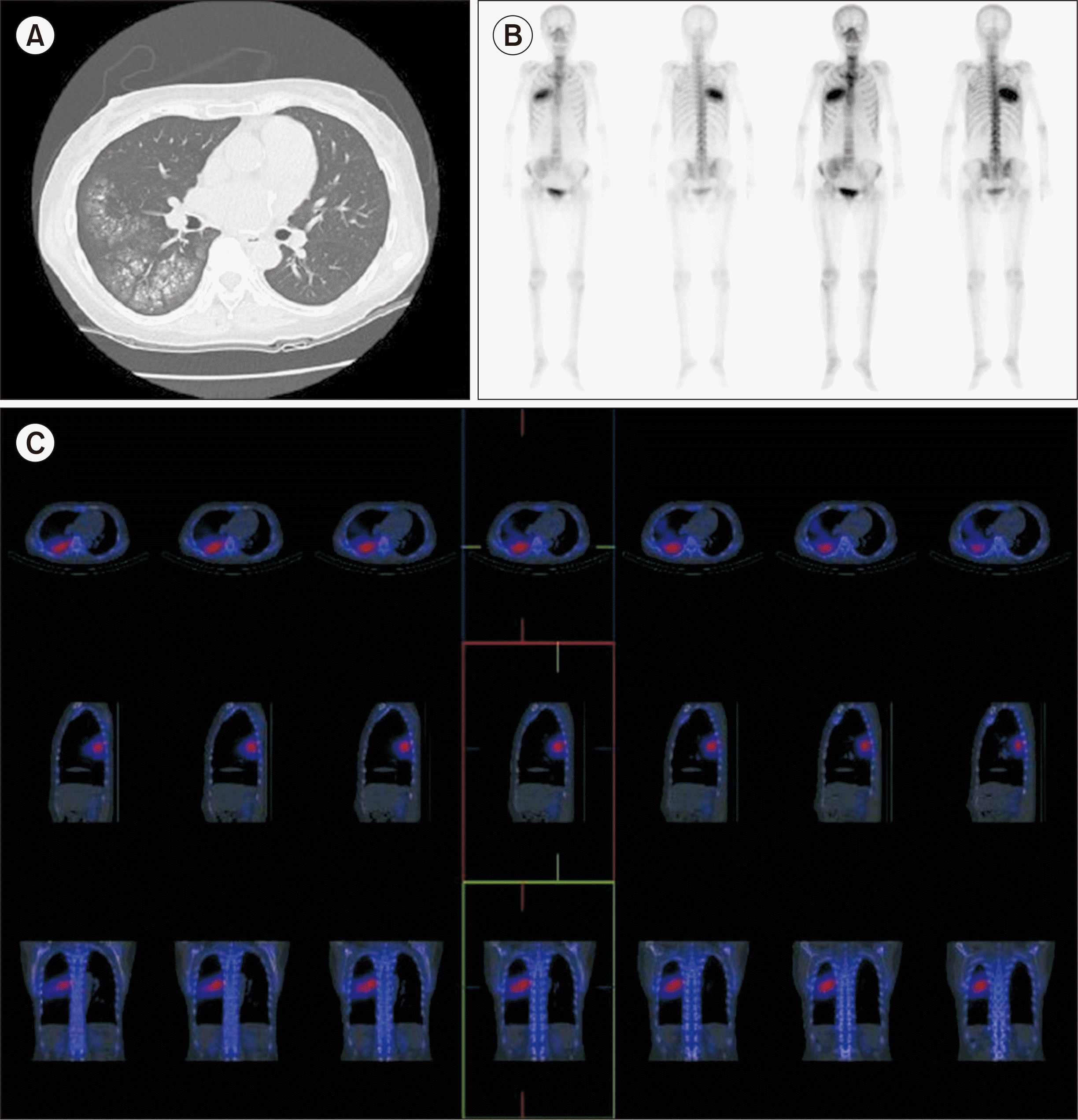
At 4.5 years (54 months) after KT, we incidentally found an abnormal lesion on the patient’s right lung in a routine chest X-ray (CXR) examination (Fig. 1). She did not have any symptoms such as dyspnea or cough. On low-dose chest computed tomography, multiple high-density centrilobular nodules in the right middle lobe (RML) and right lower lobe (RLL) were found, suggesting possible MPC (Fig. 2). Blood chemistry results showed a serum creatinine level of 0.65 mg/dL, a calcium level of 9.4 mg/dL, a phosphorus level of 2.8 mg/dL, and an iPTH level of 75.92 pg/mL. Parathyroid hormone (PTH)-related peptide was WNL. Thus, both medications for osteoporosis (bisphosphonate and vitamin D) were discontinued.
Since the patient continued to take warfarin for atrial fibrillation, we decided not to perform a lung biopsy. Instead, we performed a whole-body bone scan, the most sensitive tool for detecting MPC. The bone scan revealed multiple intense accumulation of technetium-99m-hydroxymethylene diphosphonate (Tc-99m HDP) in the RML and RLL, suggesting MPC (Fig. 2). Therefore, we confirmed the diagnosis of MPC.
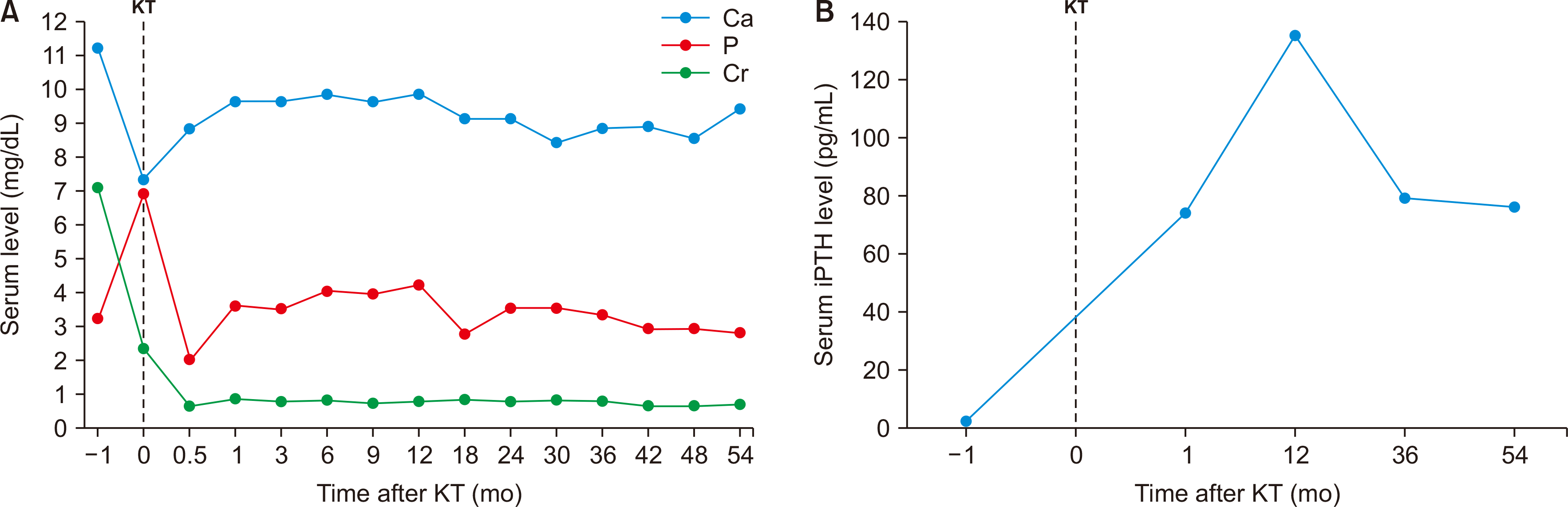
We reviewed the patient’s pretransplant blood chemistry results retrospectively. The results were as follows: serum calcium level, 11.2 mg/dL; phosphorus level, 3.2 mg/dL; iPTH level, <2.5 pg/mL; and 24-hour urine calcium excretion, 226.0 mg/day. Her serum bone-specific alkaline phosphatase (ALP) level was 18.09 µg/L (normal range, 2.9–14.5 µg/L for premenopausal women; 3.8–22.6 µg/L for postmenopausal women), which was measured only once, a month before the transplantation. As she was postmenopausal, her bone ALP was within normal ranges. However, after the transplantation, her serum calcium, phosphorus, creatinine, and iPTH levels had always been WNL, making it difficult for us to expect the occurence of MPC (Fig. 3). One possible explanation is that she probably had hidden adynamic bone disease (ABD). Treatment for osteoporosis, including bisphosphonate and vitamin D supplementation, might have contributed to calcification, leading to MPC. Therefore, we discontinued bisphosphonate and vitamin D. We also plan to regularly examine her CXR and blood chemistry results, including calcium, phosphorus, and iPTH, to monitor whether the calcification becomes aggravated.
Although the pathogenesis of MPC is poorly understood, there are several possible known risk factors. MPC is associated with an increase in the calcium-phosphate product. Metastatic calcification can develop when the calcium-phosphate product exceeds 70 mg2/dL2 [3]. Hyperparathyroidism is also a risk factor for MPC [1]. Vitamin D supplementation may induce calcification, as hypervitaminosis D is known to be related to calcification [7]. The most common cause of MPC is known to be ESRD requiring dialysis.
Generally, MPC in ESRD patients is caused by calcium-phosphate imbalance, secondary hyperparathyroidism, and acid-base imbalance [2]. Most of these conditions resolve after KT [6]. Thus, new-onset MPC among KT recipients (KTRs) is very rare.
The clinical manifestations of MPC are usually minimal, and the condition is sometimes even asymptomatic. However, MPC occasionally causes dyspnea and chronic, non-productive cough [8]. CXR examinations usually demonstrate patchy airspace opacities, but they are not very useful for diagnosing MPC. High-resolution computed tomography (HRCT) is more sensitive in detecting small amounts of calcification. The most common finding on HRCT is centrilobular ground-glass nodular opacities with numerous fluffy nodules [7]. MPC can be diagnosed via lung biopsy. Strong hematoxylin staining in the alveolar septa and walls of pulmonary vessels and bronchi is its characteristic. Instead of performing biopsy, nuclear imaging with technetium-99m-methylene diphosphonate, such as in a bone scan, can be used for the diagnosis of MPC. This could be the most sensitive tool for the early detection of MPC [9].
Nonprogressive or asymptomatic MPC does not require intervention [1]. However, MPC can sometimes become aggravated, leading to irreversible lung damage and respiratory failure. Thus, it is crucial to always pay attention to unexplained radiographic changes in ESRD patients and KTRs. The optimal treatment for MPC is not known. Nevertheless, attempts to normalize calcium and phosphorus levels have been viewed as the key to treatment.
In our case, the patient was asymptomatic and she showed no abnormal laboratory findings. However, a routine check-up CXR examination incidentally showed possible MPC. Instead of performing a lung biopsy, we successfully confirmed MPC using a bone scan without performing any invasive procedures. Therefore, when MPC is suspected, a non-invasive imaging study rather than an invasive lung biopsy should be considered first.
ABD, which is characterized by low-bone turnover resulting from a reduced number of osteoclasts and osteoblasts, is commonly found in ESRD patients [10]. Basically, either the PTH resistance in bone metabolism or oversuppression of PTH release is known to be the cause of ABD. While bone biopsy remains the gold standard in diagnosing ABD, serological markers can be helpful in guiding clinicians to confirm the diagnosis [11].
Based upon the laboratory findings, we concluded that the patient in our case possibly had hidden ABD. Thus, vitamin D supplementation leading to hypervitaminosis D could have induced hypercalcemia and calcification in sequence. Moreover, bisphosphonate addition could have diminished the bone’s calcium uptake, thereby worsening ABD [12]. Such aggravation of ABD together with hypervitaminosis D might have ultimately resulted in MPC.
In conclusion, even when blood chemistry results such as serum calcium, phosphorus, and iPTH are WNL, physicians should consider the possibility of underlying ABD in KTRs and need to be careful when prescribing bisphosphonate or vitamin D to KTRs. In addition, routine follow-up CXR examinations should be performed.
ACKNOWLEDGMENTS
Conflict of Interest
Chul Woo Yang is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflict of interest relevant to this article was reported.
REFERENCES
1. Chan ED, Morales DV, Welsh CH, McDermott MT, Schwarz MI. 2002; Calcium deposition with or without bone formation in the lung. Am J Respir Crit Care Med. 165:1654–69. DOI: 10.1164/rccm.2108054. PMID: 12070068.
2. Davies MR, Hruska KA. 2001; Pathophysiological mechanisms of vascular calcification in end-stage renal disease. Kidney Int. 60:472–9. DOI: 10.1046/j.1523-1755.2001.060002472.x. PMID: 11473629.
3. Kuzela DC, Huffer WE, Conger JD, Winter SD, Hammond WS. 1977; Soft tissue calcification in chronic dialysis patients. Am J Pathol. 86:403–24. PMID: 836675. PMCID: PMC2032079.
4. Sun HM, Chen F, Yin HL, Xu XY, Liu HB, Zhao BL. 2017; Rapid development of metastatic pulmonary calcifications in primary hyperparathyroidism: a case report and literature review. Diagn Pathol. 12:38. DOI: 10.1186/s13000-017-0628-1. PMID: 28482911. PMCID: PMC5423015.
5. Katzenstein AA, Askin FB. 1982; Surgical pathology of non-neoplastic lung disease. Major Probl Pathol. 13:1–430. DOI: 10.1038/modpathol.3880056. PMID: 7087547.
6. Wesseling-Perry K, Bacchetta J. 2011; CKD-MBD after kidney transplantation. Pediatr Nephrol. 26:2143–51. DOI: 10.1007/s00467-011-1829-6. PMID: 21394466. PMCID: PMC3203246.
7. Thurley PD, Duerden R, Roe S, Pointon K. 2009; Case report: rapidly progressive metastatic pulmonary calcification: evolution of changes on CT. Br J Radiol. 82:e155–9. DOI: 10.1259/bjr/87606661. PMID: 19592398.
8. Guermazi A, Espérou H, Selimi F, Gluckman E. 2005; Imaging of diffuse metastatic and dystrophic pulmonary calcification in children after haematopoietic stem cell transplantation. Br J Radiol. 78:708–13. DOI: 10.1259/bjr/74299224. PMID: 16046422.
9. Faubert PF, Shapiro WB, Porush JG, Chou SY, Gross JM, Bondi E, et al. 1980; Pulmonary calcification in hemodialyzed patients detected by technetium-99m diphosphonate scanning. Kidney Int. 18:95–102. DOI: 10.1038/ki.1980.114. PMID: 7218663.
10. Brandenburg VM, Floege J. 2008; Adynamic bone disease-bone and beyond. NDT Plus. 1:135–47. DOI: 10.1093/ndtplus/sfn040. PMID: 25983860. PMCID: PMC4421169.
11. Sprague SM. 2000; The role of the bone biopsy in the diagnosis of renal osteodystrophy. Semin Dial. 13:152–5. DOI: 10.1046/j.1525-139x.2000.00042.x. PMID: 10833774.
12. Amerling R, Harbord NB, Pullman J, Feinfeld DA. 2010; Bisphosphonate use in chronic kidney disease: association with adynamic bone disease in a bone histology series. Blood Purif. 29:293–9. DOI: 10.1159/000276666. PMID: 20090316.
Fig. 1
Chest X-ray (CXR) of the patient. (A) CXR taken 1 year after kidney transplantation (KT) shows no abnormal findings. (B) CXR taken 4.5 years after KT shows metastatic pulmonary calcification in the right middle lobe.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download