INTRODUCTION
Pain has protective roles in survival and nociception is an adaptive and transient process during homeostasis. However, strong and repetitive primary afferent stimuli delivered to the spinal cord can induce dysregulation of nociception, including neuroplastic changes and activation of neuroglia in the spinal cord leading to pain hypersensitivity as well as spinal or central sensitization. Therefore, the activation of microglia and astrocytes is a hallmark of spinal sensitization and chronic pain [
1–
3].
Descending serotonergic pathways originating from the brain stem exert either inhibitory or excitatory effects on the spinal nociceptive transmission [
4,
5]. In a study using acute brain slices, 5-hydroxytryptamine (5-HT) was detected by microglia and enhanced the response to acute injury [
6], suggesting that the descending serotonergic pathway could affect the activation of neuroglia during spinal sensitization. However, studies on the role of the spinal serotonergic pathway in the development of neuroglial activation are limited, and the results are inconsistent, possibly due to the differences in the pain models and 5-HT receptor subtypes activated [
7–
9]. In addition, most of the studies have focused on the effect of 5-HT receptor blockade or depleting 5-HT, but little is known about the effect of an excess on pain hypersensitivity and neuroglial activation.
This study explored the overall role of descending serotonergic pain modulating pathways in the activation of neuroglia and pain behavior by examining how an excess or deficit of spinal 5-HT affects the intensity of the mechanical allodynia and neuroglia activation in a rodent inflammatory pain model of carrageenan (CA) inflammation.
Go to :

MATERIALS AND METHODS
1. Experimental animals and intrathecal catheterization
All experiments were performed following the International Association for the Study of Pain guidelines for the Use of Animals in Research, and the experimental protocol was approved by the Institutional Animal Care and Use Committee of Chonnam National University (CNU IACUC-H-2017-10).
Male Sprague–Dawley rats (weight 220–250 g) were acclimated to the laboratory environment for 3 days before use. All animals had free access to a standard rat diet and tap water. The room temperature was maintained at 20°C–23°C with an alternating 12-hour light/dark cycle.
Under general anesthesia with sevoflurane, a polyethylene tubing with inner and outer diameters of 0.28 and 0.64 mm (PE-10 catheter; Becton Dickinson Co., Sparks, MD) was inserted into the intrathecal (i.t.) space through the atlanto-occipital membrane [
10]. The PE-10 catheter was stretched out to reduce the size before use and advanced in a caudal direction until it reached the lumbar enlargement. The other end of the PE-10 catheter was exteriorized to the top of the head and plugged with a stainless-steel wire to prevent clogging of the catheter and later administration of experiment agents. Following i.t. catheterization, the animals were allowed to recover in individual cages for 5 days before further experiments.
2. Behavioral testing and CA inflammation
Allodynic responses to mechanical stimuli were measured using the von Frey test. Animals were given 15 minutes to acclimate to a cage with a wire mesh floor. Filaments with forces of 0.41–15.2 g were applied perpendicular to the middle of the plantar surface through the wire mesh floor. Each application was maintained for 5 seconds or until paw lifting or licking, which were considered positive responses. The filament with a force of 2 g was used first, and then the force was selected by the up-and-down paradigm to determine the paw withdrawal threshold of 50% probability (PWT) [
11]. Rats having a PWT < 10 g were excluded from the study. The behavioral testing was performed by a researcher blinded to the previous treatment.
Inflammatory pain was induced by subcutaneously injecting CA (100 μL, degraded λ-CA; Wako Pure Chemical Industries, Osaka, Japan). After the PWT was measured (baseline PWT), rats received a brief inhalation of sevoflurane and then 2% CA was injected subcutaneously into the center of the plantar surface of the left hind paw. The PWT was measured every hour for 0–4 hours (early phase of CA inflammation) and 24–28 hours (late phase) after the CA injection.
3. Drugs
Serotonin in the spinal cord was depleted by i.t. administration of 5,7-dihydroxytryptamine (5,7-DHT; Sigma-Aldrich, St. Louis, MO), which has been shown to ablate serotonergic nerve fibers [
12]. Desipramine (30 mg/kg; Sigma-Aldrich) was injected intraperitoneally 45 minutes before i.t. injection of 5,7-DHT to prevent non-specific uptake of 5,7-DHT by the noradrenergic nerve fibers. The dose of 5,7-DHT was 60 μg/20 μL, which has been reported to deplete significantly endogenous spinal 5-HT. Every i.t. administration of the experimental agent was followed by flushing the catheter with 10 μL of vehicle.
To increase the concentration of 5-HT in the spinal cord, 100 μg/10 μL of 5-HT was injected 3 times at 24-hour intervals. CA or saline was injected 10 minutes after the third injection of 5-HT on day 3 (
Fig. 1).
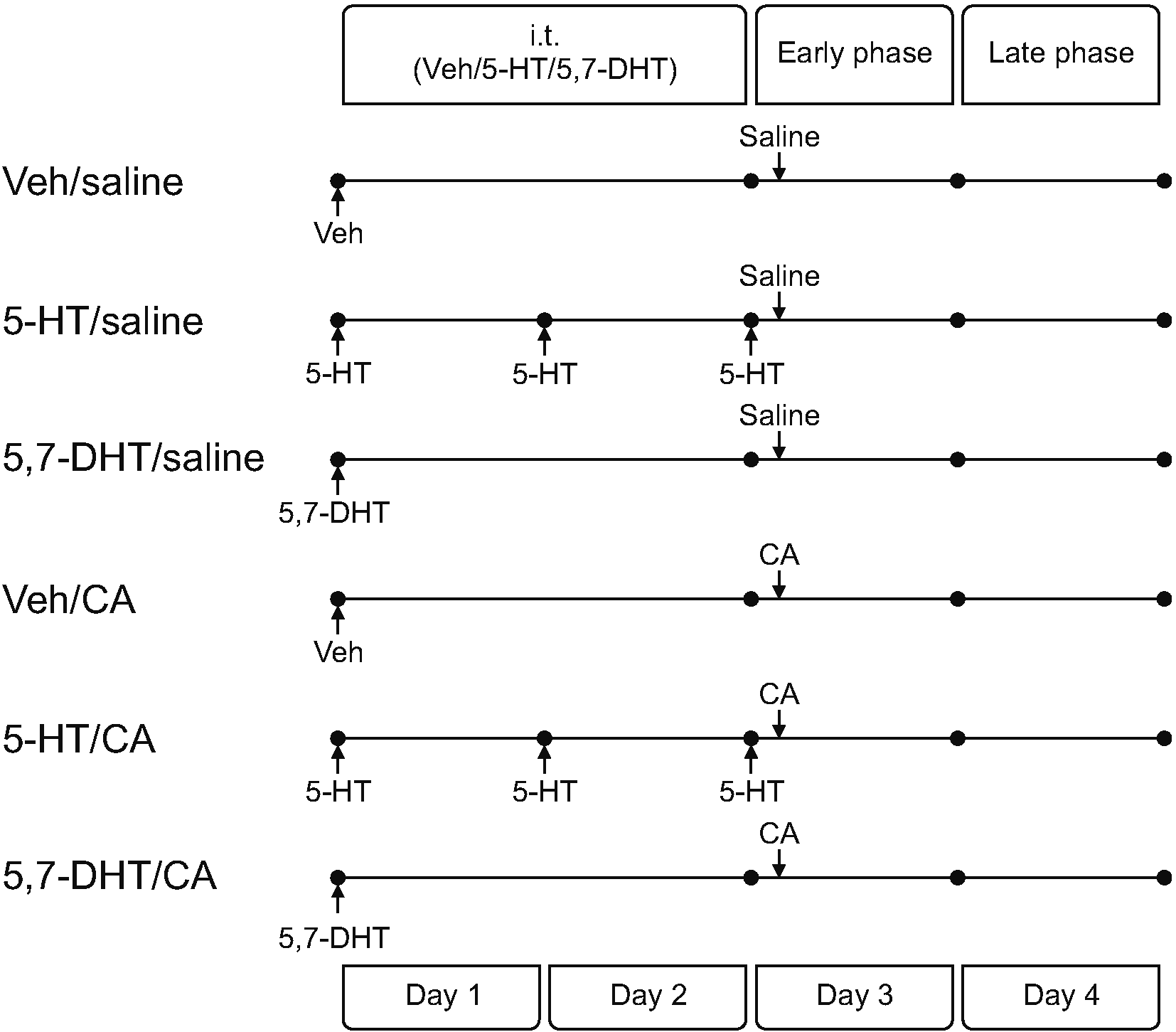 | Fig. 1Groups of animals according to the intrathecal (i.t.) treatment and the intraplantar injection. On day 1, intrathecal 5-hydroxytryptamine (5-HT), 5,7-dihydroxytryptamine (5,7-DHT) or vehicle (Veh) was administered to induce an excess, deficit or no change in the 5-HT concentration in the spinal cord, respectively. On day 3, those animals were given the intraplantar injection with carrageenan (CA) or saline. With these, the animals were classified into 6 groups: No inflammation (Veh/Saline, 5-HT/Saline, 5,7-DHT/Saline) and CA inflammation (Veh/CA, 5-HT/CA, 5,7-DHT/CA). 
|
After the 5,7-DHT or 5-HT i.t. injections, motor and sensory testing were performed daily as performed after implanting the i.t. catheter. Animals without sensory or motor deficits were used for behavioral study or immunofluorescences studies. None of the rats showed motor or sensory deficits on the day of the 5,7-DHT injection, but two rats treated with 5,7-DHT developed hemorrhagic eye wax and a watery discharge from the nose the next day after the 5,7-DHT injection and were killed immediately and excluded from the study.
4. Experiment protocol
Intrathecal administration with 5-HT, 5,7-DHT, or vehicle was started on day 1 to induce an excess, deficit, or no change in the 5-HT concentration in the spinal cord, respectively. The injection of 5-HT was performed on day 2 and day 3, but 5,7-DHT was not injected on day 2 or day 3, and instead vehicle was injected. Then, the animals were injected subcutaneously with 2% CA or saline 10 minutes after the third injection of 5-HT or vehicle on day 3 (
Fig. 1).
Two sets of experiments were performed. First, the von Frey test was performed before (baseline), 1–4 hours (early phase), and 24–28 hours (late phase) after the CA injection to assess the change in the PWT. Immunofluorescence of the dorsal horn of the spinal cord was performed at 3 hours and 27 hours after the CA injection. In the 2nd set of experiments, additional i.t. 5-HT (100 μg/10 μL) was administered at the beginning of the late phase in the Veh/Saline, Veh/CA, 5-HT/CA, and 5,7-DHT/CA groups. With this i.t. treatment, the authors investigated whether the inhibitory effect could be produced in rats with an excess or deficit of 5-HT compared with the results of a previous study which showed an anti-allodynic effect of i.t. 5-HT during the late-phase of CA inflammation in normal animals.
5. Immunofluorescence
Rats were deeply anesthetized with sevoflurane, and transcardially perfused with 0.9% sterile saline followed by 4% paraformaldehyde in 0.1 M phosphate buffered saline (PBS, pH 7.4). The lumbar enlargement of the spinal cord was dissected out and removed, then post-fixed in the 4% paraformaldehyde at 4°C overnight. After fixation, the lumbar enlargement was immersed in 30% sucrose in 0.1 M PBS at 4°C until the tissues sink down. The tissue was frozen in optimal cutting temperature compound on dry ice and then sectioned transversely on a cryostat at a 15 μm thickness. The slices were first blocked in 1% bovine serum albumin containing 0.3% Triton-X100 for 45 minutes at room temperature and then incubated overnight at 4°C with the following primary antibodies: Iba-1 (Rabbit, 1:1,000; Wako, Osaka, Japan) and GFAP (Rabbit, 1:1,000, Dako, Carpentaria, CA). The slice was washed with PBS the next day and incubated with secondary antibodies (Alexa Fluor 488, donkey-anti-rabbit, 1:500, Alexa Fluor 594; Jackson ImmunoResearch, West Grove, PA).
Images of the dorsal horn of the lumbar spinal cord were obtained using a fluorescence microscope (EVOS M5000; Thermo Fisher Scientific, Waltham, MA) at 100×. To compare fluorescent intensity, the percentage of the area of the stained cells in the dorsal horn was calculated using ImageJ (National Institutes of Health, Bethesda, MD).
6. Statistical analysis
All data are presented as mean ± standard deviation and analyzed using SPSS software ver 22.0 (SPSS Inc., Chicago, IL). The changes in mechanical allodynia (
Figs. 2,
3) were analyzed using the area under the curve (AUC) which was obtained from the time-course curve of PWT (g).
 | Fig. 2Effects of 5-HT excess or deficit in the spinal cord on the mechanical allodynia in early and late phase of carrageenan (CA) inflammation. Time course of paw withdrawal threshold (PWT) in early (A) and late phase (B) is shown in upper panel, and the corresponding area under curve (AUC) is depicted in lower panel (C and D). No significant changes are seen in the PWT and AUC in group with no inflammation (Veh/Saline, 5-HT/Saline, 5,7-DHT/Saline), but significant decrease in PWT and AUC was found in animals with CA inflammation (group Veh/CA, 5-HT/CA, 5,7-DHT/CA). The error bars indicate mean ± standard deviation. 5-HT: 5-hydroxytryptamine, 5,7-DHT: 5,7-dihydroxytryptamine, Veh: vehicle. * indicates P < 0.001 vs. group Veh/Saline, #P < 0.05 vs. group Veh/CA. 
|
 | Fig. 3Effect of additional intrathecal (i.t.) treatment of 5-HT at 24 hours after carrageenan (CA) injection into the hind paw. Time course of paw withdrawal threshold (PWT) and its corresponding area under curve (AUC) in early (A, C) and late phase (B, D) is shown. No significant changes in mechanical allodynia was produced in animals with normal level of spinal 5-HT (group Veh/CA) as well as excess and deficit of 5-HT (group 5-HT/CA and 5,7-DHT/CA). The error bars indicate mean ± standard deviation. 5-HT: 5-hydroxytryptamine, 5,7-DHT: 5,7-dihydroxytryptamine, Veh: vehicle. * indicates P < 0.001 vs. group Veh/Saline, #P < 0.001 (group 5-HT/CA) or P = 0.004 (group 5,7-DHT/CA) vs. group Veh/CA. 
|
Differences between the groups were analyzed by one-way analysis of variance followed by Tukey’s post hoc comparison test. Comparison of the AUC between the early and late phases was performed using the independent t-test. A P value < 0.05 was considered significant.
Go to :

RESULTS
Subcutaneous injection of 2% CA into the hind paw induced mechanical allodynia, as well as a significant decrease in the PWT. This decrease in the PWT was observed as early as 1–4 hours (early phase) and lasted more than 24 hours (late phase) after the CA injection (
Fig. 2A, B). Accordingly, the AUC of the Veh/CA group (normal animals) decreased significantly compared to that in the Veh/Saline group (naive animals) (
Fig. 2C, D). Animals treated with either repeated 5-HT or a single 5,7-DHT injection, but that were not injected with CA (group 5-HT/Saline and 5,7-DHT/Saline) did not show significant changes in the PWT throughout the observation period, similar to those in the Veh/Saline group.
Three repeated i.t. injections of 5-HT (group 5-HT/CA) did not affect the AUC during the early phase compared to that in the Veh/CA group (
Fig. 2C). The AUC of the 5,7-DHT/CA group was significantly lower than that of the Veh/CA group (
P = 0.004). However, in the 2nd set of experiments, no significant difference in the AUC (
P = 0.898) was detected between the Veh/CA and the 5,7-DHT/CA groups (
Fig. 3A, C).
In the late phase, the intensity of mechanical allodynia was significantly reduced in the Veh/CA group (
Fig. 2B) compared to that during the early phase (
P < 0.001). In contrast, a significant increase or no changes in the intensity were observed in the 5-HT/CA and 5,7-DHT/CA groups (
P = 0.043 and
P = 0.801, respectively). Accordingly, mechanical allodynia was significantly more severe in the 5-HT/CA and 5,7-DHT/CA groups than in the Veh/CA group (
Fig. 2B, D).
The second set of experiments was performed to clarify the role of an excess or deficit of 5-HT during the late phase, as a previous study showed that i.t. treatment with 5-HT during the late phase ameliorates the mechanical allodynia in normal animals. Intrathecal 5-HT (100 μg) was given to the Veh/CA, 5-HT/CA and 5,7-DHT/CA groups after measuring the PWT at 24 hours, but the additional i.t. treatment did not significantly change the intensity of mechanical allodynia compared to that of early phase in the all groups. However, the intensity of 5-HT/CA and 5,7-DHT/CA group was significantly lower than that of Veh/CA group (
Fig. 3B, D).
The immunofluorescence study of the dorsal horn of the spinal cord performed 3 hours (early phase) or 27 hours (late phase) after the CA injection showed that microglial activation, as measured by the percentage area of anti-Iba-1 (+) cells, was not different in the 5-HT/Saline and 5,7-DHT/Saline groups compared to the Veh/Saline group (
Fig. 4). In contrast, microglia were activated during the early phase in all groups after the CA injection compared to the Veh/Saline group. The percentage area of the Iba-1 (+) cells was higher in the 5-HT/CA and 5,7-DHT/CA groups than in the Veh/CA group.
 | Fig. 4Immunofluorescence study for microglial activation in the early and late phase of carrageenan (CA) inflammation in the dorsal horn of lumbar spinal cord. The sections are acquired 3 (A, early phase) and 27 hours (B, late phase) after carrageenan injection, and the images represent the average fluorescent intensity obtained from 4 animals in each group. The percentage of the area of Iba-1(+) cell is presented in the lower panel (C, D). The error bars indicate mean ± standard deviation. 5-HT: 5-hydroxytryptamine, 5,7-DHT: 5,7-dihydroxytryptamine, Veh: vehicle. * indicates P < 0.001 vs. group Veh/Saline, #P < 0.001 vs. group Veh/CA. Scale bar: 400 μm. 
|
Microglia were continually activated during the late phase in the Veh/CA group, but no significant changes were observed in the groups with no inflammation. The 5-HT/CA and 5,7-DHT/CA groups had a higher level of activation than the Veh/CA group (
Fig. 4). No significant difference in the area of anti-GFAP (+) immunofluorescent reactivity of astrocytes was detected among the groups, suggesting no significant involvement of astrocytes in CA inflammation (data not shown).
Go to :

DISCUSSION
In the current study, both an excess and a deficit of spinal 5-HT increased the intensity of mechanical allodynia and enhanced activation of the microglia in the spinal cord during inflammation caused by CA, suggesting that an imbalance in the spinal serotonergic pathway led to dysregulation of nociceptive processing and aggravated spinal sensitization.
Previous studies have shown that the involvement of the spinal serotonergic system in acute noxious and inflammatory pain may not be uniform. Formalin-evoked hyperalgesia during the first phase was not affected by depleting 5-HT with 5,7-DHT, but hyperalgesia diminished during the second phase [
13–
15]. Mechanical allodynia was not affected by the 5,7-DHT treatment on the paw incision test, but the analgesic effect of nefopam decreased in response to the 5,7-DHT treatment [
16].
In the current study, the intensity of mechanical allodynia during the early phase of CA inflammation was not significantly affected by the excess spinal 5-HT induced by i.t. administration of 5-HT for 3 consecutive days. A similar finding was reported in a previous study showing no anti-allodynic effect after a single i.t. injection of 5-HT during the early phase [
17]. However, the effect of i.t. 5,7-DHT on mechanical allodynia during the early phase was not consistent in the current study, as increased intensity (first set of experiments) or no effect (second set of experiments) was shown. A previous study demonstrated an aggravation of mechanical allodynia in rats during 5-HT depletion by a single i.t. 5,7-DHT injection [
15]. However, the extent of the increase in the intensity was relatively small in those animals.
These results suggest that the involvement of the spinal serotonergic pathway is limited or not crucial for acute nociceptive processing during the early phase of CA inflammation. In addition, i.t. treatment with 5,7-DHT alone or with repeated 5-HT injections without CA had no significant effect on the PWT in the von Frey test in the current study. Consistently, 5-HT-depleted rats did not show different responses to acute noxious mechanical or thermal stimuli [
8,
18–
20].
The mechanical allodynia during the late phase was partly attenuated in normal animals (group Veh/CA) compared to that during the early phase. In contrast, animals treated with repeated 5,7-HT injections or a single 5,7-DHT injection (group 5-HT/CA and 5,7-DHT/CA) failed to restore the intensity to the level of normal animals. These results might be related to the state of the 5-HT excess or deficit in the spinal cord during acute noxious stimulation in the early phase, which may have facilitated the development and maintenance of spinal sensitization. In support of this, increased activation of microglia was observed during the early phase in the 5-HT/CA and 5,7-DHT/CA groups despite no significant or slight differences in mechanical allodynia.
A previous study demonstrated that i.t. 5-HT treatment during the late phase reverses the mechanical allodynia in a dose-dependent manner, suggesting an inhibitory role of spinal 5-HT during the late phase in normal rats with CA inflammation [
17]. However, the additional treatment with 100 µg of i.t. 5-HT during the late phase in the current study had no significant effect on the intensity of mechanical allodynia in animals with an excess or a deficit of 5-HT. The spinal 5-HT pathway is likely to be important for maintaining a balance of nociceptive processing at the spinal level, and an excess and deficit of spinal 5-HT could initiate and aggravate spinal sensitization during inflammation with CA.
Spinal sensitization refers to a state of derangement in nociceptive processing involving primary afferents, interneurons, and descending pain modulating pathways at the spinal level. The role of glial cells and the neuroglial interaction has been highlighted in the development and maintenance of spinal sensitization. However, little is known about the role of descending pain modulating pathways in the activation of glial cells. In the current study, microglial activation was stronger in animals with an excess or a deficit of spinal 5-HT during the early and late phases, compared to normal animals, suggesting that the development of central sensitization at the spinal level is affected by the state of the spinal serotonergic system. In addition, the finding that the activation of microglia was stronger than that in normal animals, despite no significant changes in mechanical allodynia, supports that microglia activation during the early phase may have contributed to the increase in the intensity of mechanical allodynia during the late phase. No obvious changes in astrocytes were detected, as these were activated later and lasted longer [
1].
Previous investigations have reported no significant changes in mechanical or thermal nociception in animals with depleted spinal 5-HT but without inflammation or nerve injury [
8,
18–
20]. Furthermore, the current study also demonstrated that either a deficit or an excess of spinal 5-HT did not activate neuroglia in naive animals. This result is consistent with the notion that spinal sensitization can be initiated by a combination of several factors, such as a strong noxious primary afferent stimulus and the descending pain modulating system [
21].
In contrast to the current study, selective depletion of spinal 5-HT using i.t. 5,7-DHT treatment significantly decreases the pain behavior and microglial upregulation in the dorsal horn of the lumbar spinal cord induced by traumatic brain injury in mice [
6]. These conflicting results of microglial activation and pain behavior are in line with the finding that descending serotonergic pain modulation can be inhibitory or excitatory during nociceptive processing depending on several factors including the pain model and the 5-HT receptors activated in the spinal cord. It could be partially explained by the degree of involvement of the 5-HT3 receptor, which has a major role in facilitating nociception [
7,
12]. Evidence suggests that depleting spinal 5-HT alleviates activation of the neuroglia and pain behavior in pain models, such as the L5 spinal nerve ligation or inflammation by complete Freud’s adjuvant, in which the 5-HT3 receptor is actively involved [
7,
9]. In the traumatic brain injury model, mechanical hypersensitivity was attenuated by 5,7-DHT or a 5-HT3 receptor antagonist [
6,
20]. In contrast, the spinal 5-HT3 receptor does not have a dominant role in the inflammation caused by CA [
22], suggesting that other inhibitory 5-HT receptors could be predominantly activated and involved in CA inflammation [
17].
In the present study, the concentration of 5-HT in the spinal cord was not confirmed by quantitative measure although the method of 5-HT depletion has been used in many previous studies. In addition, the method of 3 serial daily i.t. injections of 100 mcg of 5-HT for inducing 5-HT excess has not been used previously. Despite distinct changes in pain behavior and microglia being observed with this method, further investigation using a more detailed tool could confirm the relationship between the spinal 5-HT pathway and neuroglial activation.
In conclusion, imbalance in the descending 5-HT pathway of the spinal cord increased the intensity of mechanical allodynia during the late phase and enhanced activation of the microglia during the early and late phases, suggesting that the spinal 5-HT pathway plays an essential role in keeping the nociceptive processing in balance between facilitation and inhibition during the inflammatory pain caused by CA inflammation.
Go to :









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download