Abstract
Background
The authors aimed to compare the effects of a one-time ultrasound (US)-guided subacromial corticosteroid injection and three-time ozone (O2-O3) injection in patients with chronic supraspinatus tendinopathy.
Methods
Participants were randomly assigned to the corticosteroid group (n = 22) or ozone group (n = 22). Injections in both groups were administered into subacromial bursa with an US-guided in-plane posterolateral approach. Primary outcome measure was the change in the Western Ontario Rotator Cuff Index (WORC) score between baseline and 12-weeks post-injection. Secondary outcome measures included visual analog scale and Shoulder Pain and Disability Index scores. Assessments were recorded at baseline, and 4-weeks and 12-weeks post-injection.
Results
Forty participants completed this study. Based on repeated measurement analysis of variance, a significant effect of time was found for all outcome measures in both groups. Both the groups showed clinically significant improvements in shoulder pain, quality of life, and function. Baseline, 4-week post-injection, and 12-week post-injection WORC scores (mean ± standard deviation) were 57.91 ± 18.97, 39.10 ± 20.50 and 37.22 ± 27.31 in the corticosteroid group, respectively and 69.03 ± 15.89, 39.11 ± 24.36, and 32.26 ± 24.58 in the ozone group, respectively. However, no significant group × time interaction was identified regarding all outcome measures.
Conclusions
Three-time ozone injection was not superior to a one-time corticosteroid injection in patients with chronic supraspinatus tendinopathy. It might be as effective as corticosteroid injection at 4-weeks and 12-weeks post-injection in terms of relieving pain and improving quality of life and function.
Shoulder pain, the most frequent cause of which is rotator cuff tendinopathy (RCT), constitutes approximately 16% of all musculoskeletal symptoms [1,2]. RCT, which encompasses different shoulder conditions affecting subacromial structures such as subacromial bursitis, rotator cuff tendinosis/tendinitis, and shoulder impingement syndrome, is a chronic overuse or degenerative disorder without active inflammation [2–5].
Conservative treatment of RCT includes exercise therapy, nonsteroidal anti-inflammatory drugs, physical therapy modalities, and injections such as corticosteroid and platelet-rich plasma [6–8]. To improve motion and decrease pain in all stages of RCT, corticosteroid injections are often preferred as a low-cost and effective management option [9]. Nonetheless, the short-term efficacy of the corticosteroid injection has been demonstrated [10]. Corticosteroid injections can also cause tendon failure and collagen synthesis inhibition [11].
Ozone (O3), consisting of 3 oxygen atoms, is an inorganic molecule with allotrope features. It is found in the stratosphere in nature, and it can also be formed artificially by exposure to a high-voltage electric current. Currently, a medical mixture consisting of O2 and O3 is formed by a medical generator by passing pure O2 over a high-voltage gradient [12]. The use of ozone therapy (O2-O3) in musculoskeletal disorders has increased in recent years. Ozone (O2-O3) may assist in decreasing inflammation and pain by blocking phosphodiesterase-A2 and downregulating tumor necrosis factor alpha and TNFR2 [13]. Other beneficial effects of ozone (O2-O3) in the body include increased blood flow and fibroblastic activity and starting the repair process at the tissue level [14,15]. With the use of ozone (O2-O3), no allergic side effects or destructive effects on cartilage or tendons have been noted [16].
To the authors’ knowledge, there is only one study in the literature comparing the efficacy of corticosteroid and ozone (O2-O3) injections in the management of shoulder disorders [17]. The study, conducted in patients with shoulder impingement syndrome, demonstrated that a one-time corticosteroid injection provided more significant improvement in disability and pain scores than a one-time ozone injection [17]. The authors hypothesized that three-time ozone injection would improve pain, function, and quality of life more than a one-time steroid injection. Therefore, the aim was to compare the effects of ultrasound (US)-guided subacromial a one-time corticosteroid and three-time ozone (O2-O3) injection in patients with chronic supraspinatus tendinopathy.
The design of this study was a single-center, prospective, randomized clinical trial. The study was performed from January to May 2022 on patients with chronic supraspinatus tendinopathy admitted to the physical medicine and rehabilitation outpatient clinics of a tertiary hospital. Inclusion criteria were as follows: (1) aged between 18 and 70 years, (2) rotator cuff tendinosis or partial rotator cuff tear only on the supraspinatus tendon diagnosed by US or magnetic resonance imaging (MRI), (3) chronic pain in the shoulder region for more than 3 months and an increase in pain with overhead-throwing activity. Exclusion criteria were as follows: (1) a full-thickness rotator cuff tear diagnosed by US or MRI, (2) subacromial/intra-articular injections in the last 3 months, (3) contraindications of ozone (O2-O3) injection, such as pregnancy, platelet level < 50 103/µL, glucose-6 phosphate dehydrogenase deficiency, and uncontrolled hyperthyroidism (4) allergic reaction to betamethasone or lidocaine, (5) history of hepatitis, coagulopathy, or diabetes (6) history of tumor, trauma, shoulder infection, bony lesion, inflammatory rheumatic diseases, or fracture, and (7) history of brachial plexus lesion/cervical radiculopathy. Participants were not allowed to take any topical or oral analgesics for pain during the study. The study protocol and design were approved by the local Ethics Committee (approval number: E-95961207-604.01.01-6296). Clinicaltrials.gov database registration (NCT05207384) was performed for this study. To participate the study, all participants gave written informed consent.
Participants were randomly assigned to the intervention groups using a computer-generated list of numbers by one of the researchers who did not make the assessment of the participants. Outcome measures were assessed by an independent physiatrist, blinded for the assigned treatment. The participants and the injecting physiatrist were not blinded to group allocation due to the interventions’ nature (liquid versus gas). The participants were briefed not to disclose group treatment allocation throughout assessments.
Participants’ characteristics including age, sex, dominant hand, duration of symptoms, affected shoulder, and ultrasonographic features were recorded at baseline. Subacromial bursa thickness, acromiohumeral distance, and supraspinatus tendon thickness were measured by US in both groups with the shoulder in internal rotation. The thicknesses of the subacromial bursa and supraspinatus tendon were measured in the short-axis view. Measurements were taken from the thickest part of the supraspinatus tendon and the greatest fluid thickness of the subacromial bursa (Fig. 1). Acromiohumeral distance was measured by positioning the transducer on the lateral surface of the acromion’s anterior aspect along the humerus’ longitudinal axis and defined as the distance between the acromion and humeral head (Fig. 1). Symptoms of participants were assessed with the Western Ontario Rotator Cuff Index (WORC), visual analog scale (VAS) and Shoulder Pain and Disability Index (SPADI).
Injections in both groups were performed by the same physiatrist under US guidance (with a 5–12 MHz linear transducer [Logic e portable; GE Healthcare, Jiangsu, China]) and administered into the subacromial bursa with an in-plane posterolateral approach (Fig. 2). A mixture of 1 mL corticosteroid (betamethasone 3 mg/mL) and 1 mL lidocaine (20 mg) were injected using a 27-G needle in the corticosteroid injection group. Three sessions (1 session/week) of 5 mL of ozone (O2-O3) (with a concentration of 10 μg/mL in the first session, 15 μg/mL in the second session, and 20 μg/mL in the third session) were injected using a 27-G needle in the ozone (O2-O3) injection group. The ozone (O2-O3) injectate was prepared using a medical ozone generator (EVOZONE, Reutlingen, Germany). The determined ozone (O2-O3) concentration was chosen, and a 5 mL volume of ozone (O2-O3) was filled into the syringe from the generator. It was recommended that all participants perform shoulder range of motion exercises, stretching, and strengthening exercises daily.
The primary outcome measure was the change in the WORC score between the baseline and 12-weeks post-injection. Secondary outcome measures included the SPADI and VAS scores. Assessments were recorded at baseline, and at 4-weeks and 12-weeks post-injection. In the ozone (O2-O3) group, assessments were performed on the 4th week and 12th week after completion of 3 injection sessions. Participants were asked to report any side effects at each assessment. A self-administered evaluation tool, the WORC, has been used for RCT [18]. The WORC evaluates the disease-specific quality of life and upper-extremity function [18,19]. There are 21 questions in total for 5 different domains that evaluate physical symptoms, work, emotional well-being, social well-being, and sports and recreation. A score of 100-mm is applied to each question and higher scores correspond to greater problems. The validity of the WORC for use in Turkish has been shown [18]. The hundred-point VAS (from 0 [no pain] to 100 [worst pain imaginable]) was used for assessment of shoulder pain severity in the last week.
The SPADI, a self-assessment questionnaire with 13 questions, has been applied to measure pain (5 questions) and disability (8 questions) [20]. For each question, a scale varying from 0 to 100 is used in that and higher scores correspond to greater pain and disability.
The sample size was estimated by means of the G*power (V3.1) program. To achieve a power of 80% with 5% probability of type 1 error and detect a clinically significant 17% difference in the WORC score between the 2 groups, the identified sample size was 18 patients per group based on the study by Ekeberg et al. [21]. Considering the 20% probability of patient loss during the study, a total of 44 patients were planned to be included in the study.
SPSS Statistics v15.0 (SPSS Inc., Chicago, IL) was used for statistical analysis. A nonsignificant Shapiro–Wilk test showed the data distribution’s normality. Continuous data were demonstrated as the mean ± standard deviation and median (interquartile range). Percentages were given for categorical data. Nominal data analysis was conducted using the Pearson χ2 test. Mann–Whitney U-test or independent samples t-test were used for comparing two groups. Since WORC, the primary outcome, was normally distributed, comparison was made using the independent samples t-test. Repeated-measure analysis of variance (ANOVA; two-way) was used for comparison of differences across different time points within groups and between groups, and post-hoc analysis was performed with the Bonferroni test. Statistical significance level was determined as P < 0.05.
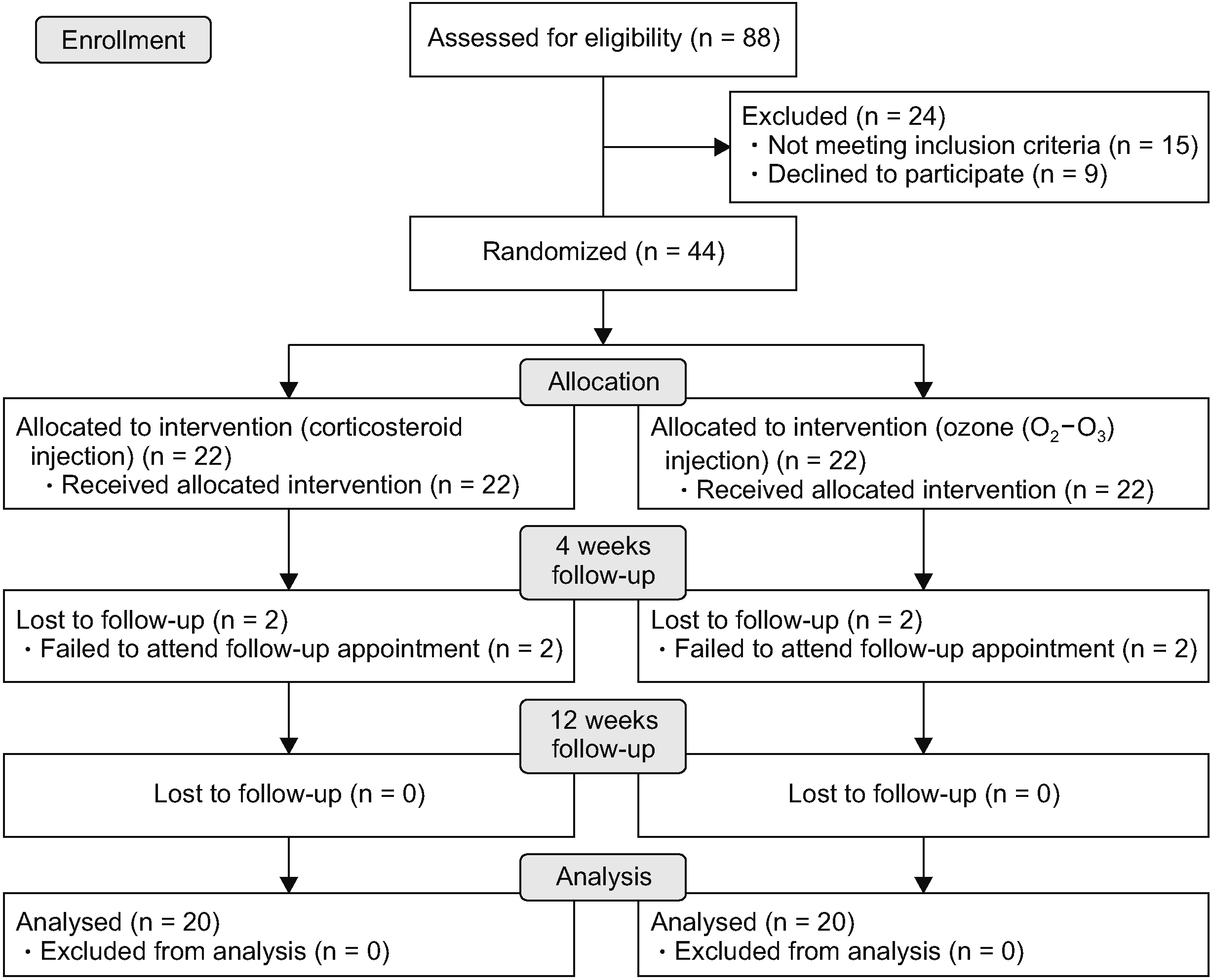
Sixty-eight patients were evaluated for study eligibility. Finally, 44 participants were randomly assigned to either the corticosteroid group or ozone (O2-O3) group. After randomization, 4 patients, 2 in the corticosteroid group and 2 in the ozone (O2-O3) group, were excluded from the study. The CONSORT diagram for participants was demonstrated in Fig. 3. There was no difference in demographic, ultrasonographic, or clinical features between the corticosteroid and ozone (O2-O3) injection groups at baseline (Table 1).
Repeated-measure analysis of variance results were presented in Table 2 and Fig. 4. A significant effect of time was detected for all outcome measures in both groups (Table 2). When baseline was compared to 4-weeks post-injection (P < 0.001 for all outcome measures) and 12-weeks-post-injection (P < 0.001 for all outcome measures), all outcome measures improved significantly over time in both groups (Fig. 4). No significant group × time interaction was identified regarding the VAS (P = 0.146), WORC (P = 0.071), and SPADI (P = 0.766) scores (Table 2, Fig. 4). The patients did not report any adverse effects in either group.
The current study aimed to compare the effects of US-guided subacromial corticosteroid and ozone (O2-O3) injection in patients with chronic supraspinatus tendinopathy. The findings of this study showed that both corticosteroid and ozone (O2-O3) injection yielded clinically significant improvements in shoulder pain, quality of life, and function. However, no significant difference was identified between a one-time corticosteroid injection and three-time ozone (O2-O3) injection.
Ozone (O2-O3) therapy has been used for treatment of various diseases since the last century. Ozone (O2-O3) injection has been demonstrated to be beneficial in the treatment of diseases such as plantar fasciitis, knee osteoarthritis, and cervical and lumbar spine pathologies [16,17,22].
There are a limited number of studies investigating the effectiveness of ozone (O2-O3) therapy in shoulder disorders [17,23,24], and the studies differ from each other in terms of the concentration and volume of injected ozone (O2-O3), the number of injection sessions, and the injection technique.
In a randomized controlled study performed on patients with shoulder impingement syndrome by Babaei-Ghazani et al. [17], corticosteroid injection was shown to improve disability and pain scores better than ozone (O2-O3) injection. In that study, unlike the present study, a session of 8 mL of ozone (O2-O3) at a concentration of 12 μg/mL was administrated into the subacromial bursa with an in-plane lateral to medial approach. The difference in the results of the present study compared to that study may be related to the repeated ozone injections at specified intervals. Repetition of ozone (O2-O3) injections at specified intervals may be required for long-term clinical improvement and continued efficacy. In tendinopathies, the International Scientific Community of Ozone Therapy (ISCO3) recommends starting with a concentration of 10 µg/mL and increasing the concentration to 20 µg/mL, if possible, in the second or third injection [25]. The ISCO3 also recommends the use of more suitable and the thinnest needle for the injection, that 5–10 mL of ozone can be injected into the shoulder tendons depending on the affected tendons, and that injections should be performed once a week up to 3–5 times [25]. Even if it might be regarded as a bias which is a limitation in the research protocol, a series of three ozone (O2-O3) injections was preferred in the present study.
The efficacy of ozone (O2-O3) injection has been demonstrated in two studies conducted in patients with RCT, involving different injection techniques and different number of sessions than ours. In these studies, similar to the present study, ozone (O2-O3) injections were performed with repeated doses at specified intervals.
In the first study conducted by Scarchilli [24] on patients with RCT, 5 sessions of 10 mL ozone (O2-O3) injection at a concentration of 10 μg/mL were performed at weekly intervals through posterior intra-articular access. In that study, it was shown that the effect of ozone (O2-O3) injections on pain control continued for more than 2 months [24].
In the second study, Moretti et al. [23] conducted a prospective randomized study on patients with supraspinatus tendinosis and compared the effectiveness of anti-inflammatory mesotherapy and ozone (O2-O3) injection. Six sessions of 10 mL of ozone (O2-O3) with a concentration of 6- to 10-μg were administered twice a week into the part with the most pain in the periarticular region. If shoulder osteoarthritis was accompanied, 10- to 15-mL of ozone (O2-O3) with a concentration of 15- to 20-μg was administered once a week intra-articularly. There was a greater decrease in VAS scores in the ozone (O2-O3) group compared to the mesotherapy group [23].
In this study, no significant difference was detected between corticosteroid and ozone (O2-O3) injection groups in terms of relieving pain and improving quality of life and function. Corticosteroid injection has already been shown to relieve pain and improve function in the short term [7]. The authors attribute the remarkable improvement in outcome measures in the ozone (O2-O3) group to the favorable biological properties of the ozone (O2-O3) molecule. Some possible effects of ozone (O2-O3) therapy in the management of musculoskeletal diseases are the start of the repair process at the tissue level and increasing the analgesic and anti-inflammatory effects and tissue oxygenation by triggering the anti-nociceptive system [12,23,26]. The results of this study show that ozone (O2-O3) injection can be considered as an alternative treatment to steroid injection in patients with supraspinatus tendinopathy. This indicates the clinical importance of the study. Considering that the follow-up period in this study was 12 weeks, further studies with longer follow-ups and different outcome measures are needed to understand whether ozone (O2-O3) is more effective than steroids in the long term.
Corticosteroid injection can cause skin hyperpigmentation or hypopigmentation, fat atrophy, and tendon rupture. Many animal models have demonstrated a decrease in rotator cuff tendon quality after corticosteroid injection [27,28]. On the contrary, ozone (O2-O3) injection is a relatively safe and effective intervention. The safety of ozone (O2-O3) injection has been shown in several studies [12,17,26]. To ensure safety, ozone (O2-O3) concentrations should be set within a specific range [12]. In this study, ozone (O2-O3) concentrations were determined by considering the concentrations recommended by the ISCO3 in tendinopathy [25]. Throughout the study, no patients experienced any adverse reactions to ozone (O2-O3) injection, demonstrating that this substance was safe in therapeutic interventions as long as the concentrations were set within a specific range. Considering the side effects of the steroid and the lack of significant side effects of ozone (O2-O3), the fact that ozone (O2-O3) is as effective as the steroid in this study also shows that ozone (O2-O3) can be alternative to steroids.
This study has some limitations. The absence of a real control group was the first limitation. It would be better to include one more group receiving no injection. The second limitation was that the injecting physiatrist and the participants could not be blinded due to the interventions’ natures. The third limitation was that three-time ozone injection versus a one-time steroid injection and the injectate volume difference between the 2 groups can be considered a bias. The fourth limitation was the limited follow-up period. The last limitation of the study was that the effect of ozone (O2-O3) injection on shoulder structures was not evaluated using an objective technique such as MRI.
In conclusion, the results of this study illustrate that three-time ozone (O2-O3) injection was not superior to a one-time corticosteroid injection in patients with chronic supraspinatus tendinopathy. It might be as effective as corticosteroid injection at 4-weeks and 12-weeks post-injection in terms of relieving pain and improving quality of life and function. Further studies with longer follow-ups are needed by involving another group having no injection.
Notes
REFERENCES
1. van der Windt DA, Koes BW, de Jong BA, Bouter LM. 1995; Shoulder disorders in general practice: incidence, patient characteristics, and management. Ann Rheum Dis. 54:959–64. DOI: 10.1136/ard.54.12.959. PMID: 8546527. PMCID: PMC1010060.
2. Lewis J, McCreesh K, Roy JS, Ginn K. 2015; Rotator cuff tendinopathy: navigating the diagnosis-management conundrum. J Orthop Sports Phys Ther. 45:923–37. DOI: 10.2519/jospt.2015.5941. PMID: 26390274.
3. Desmeules F, Boudreault J, Dionne CE, Frémont P, Lowry V, MacDermid JC, et al. 2016; Efficacy of exercise therapy in workers with rotator cuff tendinopathy: a systematic review. J Occup Health. 58:389–403. DOI: 10.1539/joh.15-0103-RA. PMID: 27488037. PMCID: PMC5356973.
4. Neer CS 2nd. 1983; Impingement lesions. Clin Orthop Relat Res. 173:70–7. DOI: 10.1097/00003086-198303000-00010. PMID: 6825348.
5. Chard MD, Cawston TE, Riley GP, Gresham GA, Hazleman BL. 1994; Rotator cuff degeneration and lateral epicondylitis: a comparative histological study. Ann Rheum Dis. 53:30–4. DOI: 10.1136/ard.53.1.30. PMID: 8311552. PMCID: PMC1005239.
6. Leong HT, Fu SC, He X, Oh JH, Yamamoto N, Hang S. 2019; Risk factors for rotator cuff tendinopathy: a systematic review and meta-analysis. J Rehabil Med. 51:627–37. DOI: 10.2340/16501977-2598. PMID: 31489438.
7. Lin MT, Chiang CF, Wu CH, Huang YT, Tu YK, Wang TG. 2019; Comparative effectiveness of injection therapies in rotator cuff tendinopathy: a systematic review, pairwise and network meta-analysis of randomized controlled trials. Arch Phys Med Rehabil. 100:336–49.e15. DOI: 10.1016/j.apmr.2018.06.028. PMID: 30076801.
8. Shin KM. 2011; Partial-thickness rotator cuff tears. Korean J Pain. 24:69–73. DOI: 10.3344/kjp.2011.24.2.69. PMID: 21716613. PMCID: PMC3111562.
9. Kwong CA, Woodmass JM, Gusnowski EM, Bois AJ, Leblanc J, More KD, et al. 2021; Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 37:510–7. DOI: 10.1016/j.arthro.2020.10.037. PMID: 33127554.
10. Buchbinder R, Green S, Youd JM. 2003; Corticosteroid injections for shoulder pain. Cochrane Database Syst Rev. 2003:CD004016. DOI: 10.1002/14651858.CD004016. PMID: 12535501. PMCID: PMC6464922.
11. Browning DG, Desai MM. 2004; Rotator cuff injuries and treatment. Prim Care. 31:807–29. DOI: 10.1016/j.pop.2004.08.004. PMID: 15544822.
12. de Sire A, Agostini F, Lippi L, Mangone M, Marchese S, Cisari C, et al. 2021; Oxygen-ozone therapy in the rehabilitation field: state of the art on mechanisms of action, safety and effectiveness in patients with musculoskeletal disorders. Biomolecules. 11:356. DOI: 10.3390/biom11030356. PMID: 33652804. PMCID: PMC7996934. PMID: c6c418c014c6416f87f37f792e1c01ad.
13. Bahrami MH, Raeissadat SA, Barchinejad M, Elyaspour D, Rahimi-Dehgolan S. 2019; Local ozone (O2-O3) versus corticosteroid injection efficacy in plantar fasciitis treatment: a double-blinded RCT. J Pain Res. 12:2251–9. DOI: 10.2147/JPR.S202045. PMID: 31413624. PMCID: PMC6661991.
14. Bocci VA. 2006; Scientific and medical aspects of ozone therapy. State of the art. Arch Med Res. 37:425–35. DOI: 10.1016/j.arcmed.2005.08.006. PMID: 16624639.
15. Biazzo A, Corriero AS, Confalonieri N. 2018; Intramuscular oxygen-ozone therapy in the treatment of low back pain. Acta Biomed. 89:41–6. DOI: 10.23750/abm.v89i1.5315. PMID: 29633741. PMCID: PMC6357609.
16. Moretti M. 2010; Can oxygen-ozone injections in sport overuse tendinopathies be a valid alternative to cortisone therapy? Int J Ozone Ther. 9:21–4.
17. Babaei-Ghazani A, Fadavi HR, Eftekharsadat B, Ebadi S, Ahadi T, Ghazaei F, et al. 2019; A randomized control trial of comparing ultrasound-guided ozone (O2-O3) vs corticosteroid injection in patients with shoulder impingement. Am J Phys Med Rehabil. 98:1018–25. DOI: 10.1097/PHM.0000000000001240. PMID: 31188145.
18. El O, Bircan C, Gulbahar S, Demiral Y, Sahin E, Baydar M, et al. 2006; The reliability and validity of the Turkish version of the Western Ontario Rotator Cuff Index. Rheumatol Int. 26:1101–8. DOI: 10.1007/s00296-006-0151-2. PMID: 16799776.
19. Basar S, Citaker S, Kanatli U, Ozturk BY, Kilickap S, Kafa NK. 2014; Assessment of function in patients with rotator cuff tears: functional test versus self-reported questionnaire. Int J Shoulder Surg. 8:107–13. DOI: 10.4103/0973-6042.145249. PMID: 25538429. PMCID: PMC4262865.
20. Roach KE, Budiman-Mak E, Songsiridej N, Lertratanakul Y. 1991; Development of a shoulder pain and disability index. Arthritis Care Res. 4:143–9. DOI: 10.1002/art.1790040403. PMID: 11188601.
21. Ekeberg OM, Bautz-Holter E, Keller A, Tveitå EK, Juel NG, Brox JI. 2010; A questionnaire found disease-specific WORC index is not more responsive than SPADI and OSS in rotator cuff disease. J Clin Epidemiol. 63:575–84. DOI: 10.1016/j.jclinepi.2009.07.012. PMID: 19836206.
22. Babaei-Ghazani A, Najarzadeh S, Mansoori K, Forogh B, Madani SP, Ebadi S, et al. 2018; The effects of ultrasound-guided corticosteroid injection compared to oxygen-ozone (O2-O3) injection in patients with knee osteoarthritis: a randomized controlled trial. Clin Rheumatol. 37:2517–27. DOI: 10.1007/s10067-018-4147-6. PMID: 29796866.
23. Moretti B, Lanzisera R, Sisti GL, Moretti L, Patella S, Patella V, et al. 2005; O2-O3 therapy in tendinopathies and entrapment syndromes. Riv Ital Ossigeno Ozonoterapia. 4:20–9.
24. Scarchilli A. 2008; Indications and limits of intra-articular oxygen-ozone therapy for rotator cuff tendinopathy. Int J Ozone Ther. 7:49–52.
25. Alexandre A, Baeza J, Kumar VS. 2014. Ozone in non-rheumatic locomotor system pathologies. International Scientific Committee of Ozone Therapy;Madrid:
26. Babaei-Ghazani A, Karimi N, Forogh B, Madani SP, Ebadi S, Fadavi HR, et al. 2019; Comparison of ultrasound-guided local ozone (O2-O3) injection vs corticosteroid injection in the treatment of chronic plantar fasciitis: a randomized clinical trial. Pain Med. 20:314–22. DOI: 10.1093/pm/pny066. PMID: 29868796.
27. Maman E, Yehuda C, Pritsch T, Morag G, Brosh T, Sharfman Z, et al. 2016; Detrimental effect of repeated and single subacromial corticosteroid injections on the intact and injured rotator cuff: a biomechanical and imaging study in rats. Am J Sports Med. 44:177–82. DOI: 10.1177/0363546515591266. PMID: 26216105.
28. Mikolyzk DK, Wei AS, Tonino P, Marra G, Williams DA, Himes RD, et al. 2009; Effect of corticosteroids on the biomechanical strength of rat rotator cuff tendon. J Bone Joint Surg Am. 91:1172–80. DOI: 10.2106/JBJS.H.00191. PMID: 19411466. PMCID: PMC7002078.
Fig. 1
(A) Measurement of the thicknesses of the subacromial bursa (1) and supraspinatus tendon (2). (B) Measurement of the acromiohumeral distance (3). A: acromion, D: deltoid, HH: humeral head, ST: supraspinatus tendon.
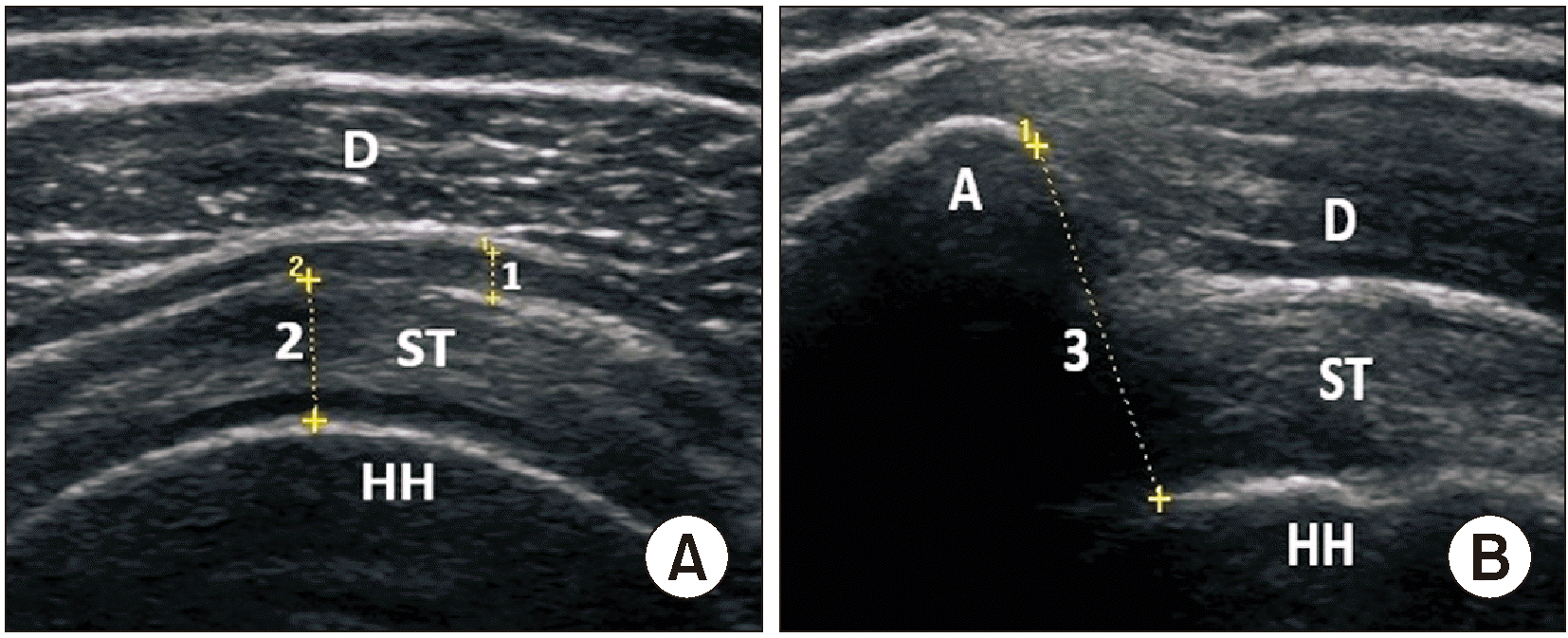
Fig. 2
Ultrasound-guided subacromial bursa injection with an in-plane posterolateral approach. Arrows: needle, A: acromion, D: deltoid, HH: humeral head, ST: supraspinatus tendon, SB: subacromial bursa, LAT: lateral.
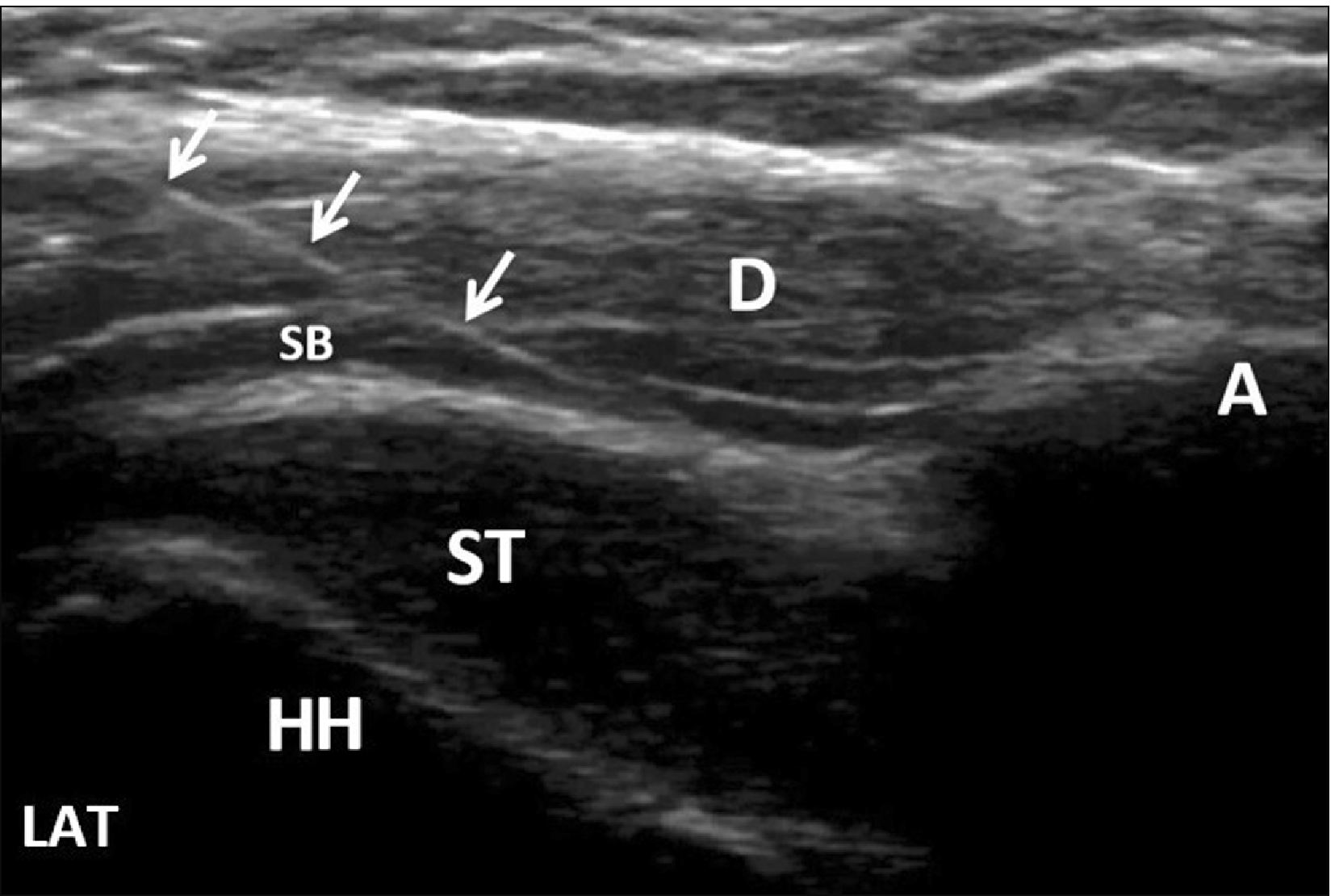
Fig. 4
Change of the outcome measures over time in corticosteroid and ozone (O2-O3) groups based on the repeated-measure analysis of variance (estimated marginal means are shown). (A) Visual analog scale (VAS). (B) The Western Ontario Rotator Cuff Index (WORC). (C) Shoulder Pain and Disability Index (SPADI).
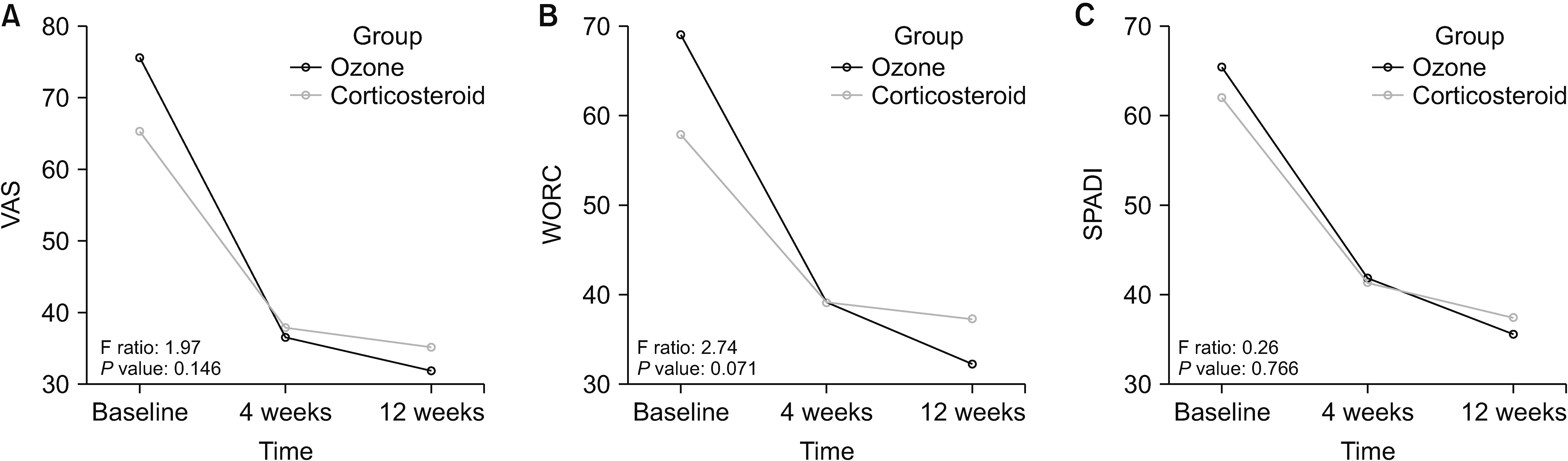
Table 1
Demographic and clinical features of the corticosteroid and ozone (O2-O3) groups at baseline
Table 2
Summary of findings for outcome measures
| Outcome measures | Score | Within-group change score | Repeated measure ANOVA F (P) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 4 weeks | 12 weeks | Baseline vs. 4 weeks | Baseline vs. 12 weeks | 4 weeks vs. 12 weeks | Time | Time × Group | |||
| VAS | ||||||||||
| Corticosteroid | 65.25 ± 15.85 | 37.75 ± 21.79 | 35.00 ± 25.80 |
–27.50 (–40.12, –14.87)* |
–30.25 (–44.64, –15,85)* |
–2.75 (–14.52, 9.02) |
61.78 (< 0.001)** | 1.97 (0.146) | ||
| Ozone (O2-O3) | 75.50 ± 17.00 | 36.50 ± 22.07 | 31.75 ± 26.42 |
–39.00 (–51.62, –26.37)* |
–43.75 (–58.14, –29.35)* |
–4.75 (–16.52, 7.02) |
||||
| WORC | ||||||||||
| Corticosteroid | 57.91 ± 18.97 | 39.10 ± 20.50 | 37.22 ± 27.31 |
–18.81 (–30.47, –7.14)* |
–20.69 (–34.27, –7.10)* |
–1.88 (–13.91, 10.15) |
38.75 (< 0.001)** | 2.74 (0.071) | ||
| Ozone (O2-O3) | 69.03 ± 15.89 | 39.11 ± 24.36 | 32.26 ± 24.58 |
–29.91 (–41.57, –18.25)* |
–36.77 (–50.35, –23.18)* |
–6.85 (–18.89, 5.18) |
||||
| SPADI | ||||||||||
| Corticosteroid | 62.00 ± 19.24 | 41.31 ± 22.56 | 37.42 ± 26.63 |
–20.68 (–33.12, –8.24)* |
–24.58 (–37.60, –11.49)* |
–3.89 (–16.70, 8.91) |
32.15 (< 0.001)** | 0.26 (0.766) | ||
| Ozone (O2-O3) | 65.38 ± 16.22 | 41.81 ± 26.54 | 35.53 ± 24.80 |
–23.57 (–36.01, –11.13)* |
–29.85 (–42.94, –16.76)* |
–6.27 (–19.08, 6.53) |
||||




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download