Abstract
Background
Most international bodies recommended against musculoskeletal steroid injection during the COVID-19 pandemic, fearing that the immunosuppressive effects of the steroid could worsen COVID-19 infection, thus prolonging the suffering of patients with severe musculoskeletal disease. The authors’ aim is to analyze the risk of COVID-19 infection after musculoskeletal injections.
Methods
This is a retrospective study of patients who visited a sports medicine clinic and received musculoskeletal steroid injections between January 1, 2020 and February 28, 2021. The collected data was compared with the national COVID-19 registry to identify positive COVID-19 patients. The patients were only considered positive for COVID-19 following corticosteroid injection within 3 months after injection.
Results
Out of 502 steroid injections; 79.7% (n = 400) received a single injection in one day, 19.1% (n = 96) received steroid injections at 2 sites in one day, and 1.2% (n = 6) received steroid injections at 3 sites in one day. Using the Fisherʼs exact test, there was no statistically significant association of COVID-19 infection between the steroid group and control group (relative risk, 1.44; 95% confidence interval, 0.9–23.1, P = 0.654). Only one patient contracted mild COVID-19 with no post COVID complications.
Musculoskeletal steroid injections are used to treat various musculoskeletal diseases: inflammatory arthritis, non-inflammatory arthritis, bursitis, tenosynovitis, and carpal tunnel syndrome [1]. Musculoskeletal steroid injections have proven benefits, including helping patients control debilitating inflammatory joint disease, improving quality of life by controlling pain, improving range of motion and facilitating an early return to work, as well as delaying or preventing surgical intervention [1]. In some cases, it aids clinicians as a diagnostic tool in diagnosing musculoskeletal disease. The use of musculoskeletal steroid injections is highly supported by evidence for acute pain control or increased function for some patients with certain musculoskeletal diseases [1,2]. Steroids are well known for their anti-inflammatory effects due to their inhibitory action on phospholipase A2, reducing prostaglandin synthesis, and reducing migration of neutrophils to the site of an injury. [3].
The recent global public health threat posed by the COVID-19 pandemic spread chaos around the world, including Malaysia. In Malaysia, the first wave started on January 25, 2020, the second wave persisted between February 27 and March 1, 2020, and the third wave of the pandemic started during late September and lasted until February 2021. There are growing concerns with widely used glucocorticoids during the COVID-19 pandemic due to the associated immunosuppressive effects, which may increase the risk of COVID-19 infection and worsen COVID-19 patient outcome. One study demonstrated the immunosuppressive effect of corticosteroid injection leading to increased risk of infection. Sytsma et al. [4] examined 15,068 patients who received musculoskeletal steroid injection during the influenza season. There was increased risk of influenza infection despite vaccination following injections (relative risk, 1.52; 95% confidence interval [CI], 1.20–1.93) and women younger than 65 years were at the highest risk.
In the early COVID-19 era, a guideline published on March 23, 2020 by the UK National Health Service recommended against the use of steroid injection unless indicated [5]. The guideline was updated on June 16, 2020, November 20, 2020, and January 21, 2021 on corticosteroid injection, stating that musculoskeletal steroid injections can be considered for severe symptoms after failed physical rehabilitation and splinting [5]. The American Society of Regional Anesthesia and Pain Medicine and the European Society of Regional Anesthesia and Pain Therapy published a joint statements on March 27, 2020, recommending the use of musculoskeletal corticosteroid injection with caution after evaluating the risks/benefits, especially in high risk patients [6]. In inflammatory arthritis, the injection is only recommended in the presence of active synovitis ± effusion, using lowest clinically effective doses (maximum 40 mg methylprednisolone/triamcinolone acetonide for large joints; 20 mg for smaller joints) [5]. For non-inflammatory arthritis, musculoskeletal steroid injection can be considered if the patient has high levels of pain and disability, failed conservative management, and where there is a significant negative effect on their wellbeing [5]. For radicular pain, musculoskeletal steroids are not recommended during the pandemic, and referral for epidural or nerve block is recommended for severe pain despite physiotherapy modalities and oral pain medications [5].
A National Survey of the Members of the British Society of Skeletal Radiologists on musculoskeletal steroid injection reported a drop of more than 80%, an increased backlog of procedures, and an increase in the wait time, which could be debilitating in managing musculoskeletal pain [7]. This study demonstrated a significant impact on the service of musculoskeletal pain management. Therefore, a retrospective survey was conducted to determine the incidence of COVID-19 following musculoskeletal steroid injection in the authors’ center and performed a risk analysis to determine risk of contracting COVID-19 following corticosteroid injections. The hypothesis was that musculoskeletal steroid injection does not confer increased risk of COVID-19 infection and does not increase the risk of severe COVID-19 infection. This research will add a significant value in the use of musculoskeletal steroid injection to treat severe debilitating musculoskeletal pain in the mist of the COVID-19 pandemic. Knowing the possible risks of contracting COVID-19 following musculoskeletal steroid injection will help change the perceptions of physicians in managing musculoskeletal disease.
This is a retrospective cohort study. This study was conducted from January 1, 2020 until February 28, 2021 during the COVID-19 pandemic and pre-COVID-19 vaccination era in Malaysia. All patients who visited the sports medicine clinic and received musculoskeletal steroid injections from January 1, 2020 to February 28, 2021 were included in this study. This period was chosen because the first statement by the World Health Organization about COVID-19 was on December 31, 2019, to the date of the first day of the Malaysian vaccination program launched, February 24, 2021.
The flow of the study is represented in a flowchart in Fig. 1. The list of musculoskeletal injection procedures in the sports medicine clinic was obtained from specific musculoskeletal injection procedure data registries kept in the sports medicine clinic. Patients who received corticosteroid injections with or without other pharmacological adjuncts were included in this study. Case note of the patients were examined using electronic medical records (EMRs). Demographic data, including age, race, and diagnosis, were included in this study. In the EMR, there were indications for musculoskeletal steroid injections, site of injections, type of steroid used, any other medication added during the injections, and complications following steroid injections. All these data were included in this study. Then, the authors compared their list with the national COVID-19 registry to identify positive COVID-19 patients. The patient was only considered positive for COVID-19 following corticosteroid injection when he/she had a positive real-time polymerase chain reaction for COVID-19 within 3 months after injection. Friedly et al. [8] studied this effect in spinal injections and found that methylprednisolone and triamcinolone injections resulted in pituitary suppression in 41% of patients at 3 weeks. Abdul et al. [9] reported hypothalamic pituitary axis (HPA) suppression in 87% at 7 days after epidural steroid injection (ESI), 43% on day 14, and 7% on day 28 using 80 mg methylprednisolone. Iranmanesh et al. [10] reported HPA suppression in up to 12 weeks using ESI with triamcinolone 80 mg. Thus, the systemic absorption of the musculoskeletal steroid lasted for a maximum of 12 weeks.
Once the patients were identified and included in this study, they were called and the purpose of the study was explained. After consent was given and signed by the patients using a Google form, they were asked regarding the date of their first positive swab, the location of the swab, as well as their date of admission, number of admission days, and history of intensive care unit admission. Any incomplete data was excluded from this study. This study was approved by the Medical Research & Ethic Committee NMRR-21-305-58854 (IIR).
Statistical analysis was performed using IBM Co. Released 2013. IBM SPSS statistics for Mac, version 27.0 (IBM Co., Armonk, NY). The steroid group and control group were then compared for any statistical significance in the number of positive cases using Fisher exact test. The null hypothesis for this study was that there is no statistically significant difference in the number of COVID-19-positive individuals in both groups. The relative risk (RR) of contracting COVID-19 following corticosteroid injections was examined, a P value < 0.05 was considered significant, and an RR of more than 1 was considered an increased likelihood of contracting COVID-19.
Out of 1,105 patients who received musculoskeletal interventions, 452 (40.9%) patients received a corticosteroid with or without other pharmacological adjuncts, while 653 (59.1%) patients received other pharmacological musculoskeletal interventions including hyaluronic acid injections, prolotherapy, platelet-rich plasma interventions, and diagnostic local anesthetic injections. The range of patients receiving steroid therapy was from 18 to 89 years old, with a mean age of 51 years old ± 16.36. Female patients made up 51.5% (n = 233) of the total, while 48.5% (n = 219) were male.
A total of 502 steroid injections were done; 79.7% (n = 400) received a single injection in one day, 19.1% (n = 96) received steroid injections at 2 sites in one day, and 1.2% (n = 6) received 3 steroid injections at 3 sites in one day. Out of 502 injections, 19.3% (n = 97) received pure steroid, 78.9% (n = 396) received a steroid mixed with local anesthetics (lignocaine or bupivacaine), 1.2% (n = 6) received a steroid mixed with hyaluronic acid, and 0.6% (n = 3) received a steroid mixed with prolotherapy. Triamcinolone was used in all procedures (100%), ranging from a total of 10 mg to 120 mg of triamcinolone per patient per day. The most common region for musculoskeletal injections were the knee (44.2%), shoulder (21.9%), and ankle (13.1%) as in Table 1. The most common diagnosis receiving corticosteroid injection was knee osteoarthritis (27.3%) as in Table 2.
Out of 452 patients, 0.4% (n = 2) had skin discoloration and 0.2% (n = 1) had nerve injury. Seven patients had a history of positive COVID-19 from national registry data, but only 1 patient had positive COVID-19 following injection, at day 60 post injection. He had mild symptoms of COVID-19: running nose, cough and flu like symptoms. He was quarantined at the quarantine center for 14 days and did not develop any complications from COVID-19. The incidence of COVID-19 infection following musculoskeletal steroid injection was 221 infections per 100,000 population.
The RR was 1.44 (95% confidence interval 0.9–23.1, P = 0.654) demonstrating that there was no significant difference between the steroid group and the control group (Table 3). Therefore, the null hypothesis of this study was not rejected.
This would be the first large study that analyzed risk of COVID-19 following musculoskeletal steroid injections. The incidence of COVID-19 following musculoskeletal steroid injection is higher (221.2 infections per 100,000 populations), as compared to Malaysia’s 7 day incidence rate of 26.6 infections per 100,000 populations between March 16, 2020 and May 31, 2021 [11]. However, the incidence is lower as compared to Bugeja et al. [12] (544 infections per 100,000 populations) and McKean et al. [13] (1,408 infections per 100,000 populations). Although the incidence of COVID-19 is high, there were no fatalities reported compared to the case fatality rate in Malaysia, which is 0.6% (CI: < 0.1, 3.7).
Azwan Aziz et al. [14] only examined 35 patients who received musculoskeletal corticosteroid injection among Malaysian population in Sabah between December 2020 and June 2020. The total triamcinolone per patient per day, ranging from 20 mg to 40 mg, was slightly lower than that used in the present study, which was 10 mg to 120 mg triamcinolone per patient per day [14]. There was no patient diagnosed with COVID-19 following corticosteroid injection in the Azwan Aziz et al. [14] study, while this study had one positive COVID-19 case who was diagnosed on day 60 post-injection. He had mild disease requiring admission to a quarantine center for 14 days for observation and did not develop severe complication of COVID-19. There were limitations identified in the Azwan Aziz et al. [14] study: (i) the small sample size reduced the confidence of the study, ii) there was no risk analysis compared to the present study, in which the outcome was statistically insignificant, and (iii) the diagnosis of COVID-19 was a self-reported diagnosis with no microbiological confirmation.
An almost similar study was from Malta, a small European country, by Bugeja et al. [12], who examined 734 patients who received musculoskeletal steroid injections from March 2020 until January 2021. This was the first study that utilized a national registry for COVID-19. Thus, the data interpretation in this study was more confident. They were using a standard dose of methylprednisolone of 40 mg per joint per day. Bugeja et al. [12] considered patients who tested positive COVID-19 within 30 days after injection, while the present study kept track of diagnoses up to 3 months after injection. The duration of the period of checking for COVID-19 after injection was slightly shorter than ours. There were 4 patients from the steroid group and 3 patients from the control group who contracted COVID-19 [12]. However, the severity of COVID-19 was not stated in this study.
A retrospective study was performed by McKean et al. [13] on 443 patients in the UK from February 2020 to June 2020 at Stoke Mandeville Hospital. They used 6.6 mg dexamethasone for nerve root injection, 40 mg triamcinolone for large joints and 40 mg methylprednisolone for small joints [13]. There were 8 patients who underwent COVID-19 screening; 4 had respiratory symptoms and 4 had routine COVID-19 screening [13]. There were no positive cases in this study. However, due to selective testing of COVID-19, data interpretation in this study should be done cautiously.
Chang et al. [15] examined 71 subjects from April 2020 to May 2020 who received image-guided steroid injections. Fifty-four percent used triamcinolone, 44% used betamethasone, 1% used methylprednisolone, and 1% used dexamethasone [15]. Chang et al. [15] used a phone survey and EMR in 91% of cases, and EMR only in 9% of cases to identify positive COVID-19 after the injections [15]. Only one patient had a diagnosis of mild COVID-19 at day 19 post-infection and did not require hospitalization [15]. The small sample size limits sufficient analysis of this study.
This is a first large study in the Asian region. This study used the national data registry for COVID-19 as a method of data collection. Thus, the risk of missing COVID-19 data was reduced significantly, as all positive COVID-19 cases would have been reported in the national registry. This empowered the value of this study. The use of EMR has created a way to examine a large database and enabled faster analysis, especially when data analysis is supremely important, as in COVID-19. This is also the first study that combined the data of a few studies from a systematic literature search to perform risk analysis. This is very important, as a small sample size could reduce the significance of the value in this study. Nonetheless, retrospective study has its limitations. Complications following corticosteroid injections may not represent their true prevalence as they may be minor, unreported cases. Thus, such cases cannot be measured depending on EMR report outcomes of injections, and significant biases may affect the outcomes of the study. On the other hand, there were no serious complications following corticosteroid injections reported in this study. Vaccination has stabilized the infectivity rate of COVID-19. However, does steroid injection reduced the effectivity of vaccine? That question has not been answered in this study. With the development of vaccines, the risk of contracting severe COVID-19 following steroid injection can be analysed between the vaccinated and unvaccinated, serving as platforms for future research.
Knowing the risk of contracting COVID-19 can reshape the dynamic of the management of musculoskeletal disease. It allows patients with this disease to get better pain management and live happier lives. From the data gathered in the authors’ systemic literature search, there were less likelihood of contracting COVID-19 following musculoskeletal injection. In fact, larger population examination did not reveal increased risk of severe COVID-19. There were no major complications reported; 0.4% (n = 2) had skin discoloration and 0.2% (n = 1) had nerve injury. There was 1 COVID-19 case reported at day 60 after injection. The case was mild and there were no complications from COVID-19. After careful risk-benefit analysis and shared decision making with patients, the authors recommend that musculoskeletal steroid injection be used in musculoskeletal pain management as it could reduce the backlog of operations and improve pain outcomes for those who suffer.
ACKNOWLEDGMENTS
I would like to thank the Director General, Ministry of Health, Malaysia, Tan sri Dato Sri Dr Noor Hisham bin Abdullah and the Head of Centre for Disease Control, Ministry of Health, Malaysia, Dr Wan Noraini binti Wan Mohamed Noor for their support and high contribution in this study.
Notes
REFERENCES
1. Stephens MB, Beutler AI, O'Connor FG. 2008; Musculoskeletal injections: a review of the evidence. Am Fam Physician. 78:971–6. PMID: 18953975.
2. MacMahon PJ, Eustace SJ, Kavanagh EC. 2009; Injectable corticosteroid and local anesthetic preparations: a review for radiologists. Radiology. 252:647–61. DOI: 10.1148/radiol.2523081929. PMID: 19717750.
3. Ericson-Neilsen W, Kaye AD. 2014; Steroids: pharmacology, complications, and practice delivery issues. Ochsner J. 14:203–7. PMID: 24940130. PMCID: PMC4052587.
4. Sytsma TT, Greenlund LK, Greenlund LS. 2018; Joint corticosteroid injection associated with increased influenza risk. Mayo Clin Proc Innov Qual Outcomes. 2:194–8. DOI: 10.1016/j.mayocpiqo.2018.01.005. PMID: 30225449. PMCID: PMC6124339.
5. National Health Service. 2020. Clinical guide for the management of patients with musculoskeletal and rheumatic conditions on corticosteroids during the coronavirus pandemic [Internet]. National Health Service;London: Available at: https://www.csp.org.uk/media/1264903.
6. Shanthanna H, Cohen SP, Strand N, Lobo CA, Eldabe S, Bhatia A, et al. Recommendations on chronic pain practice during the COVID-19 pandemic. ASRA/ESRA COVID-19 guidance for chronic pain practice [Internet]. American Society of Regional Anesthesia and Pain Medicine;Pittsburgh (PA): Available at: https://www.hlz.hr/wp-content/uploads/2020/04/asra_esra_covid-19_and_chronic_pain-1.pdf.
7. Dalili D, Fairhead R, Mermekli A, Papanikitas J, Teh J, Hughes R, et al. 2021; Impact of the COVID-19 pandemic on corticosteroid injection services: a national survey of members of the British Society of Skeletal Radiologists (BSSR). Br J Radiol. 94:20210327. DOI: 10.1259/bjr.20210327. PMID: 34520669. PMCID: PMC9328042.
8. Friedly JL, Comstock BA, Heagerty PJ, Bauer Z, Rothman MS, Suri P, et al. 2018; Systemic effects of epidural steroid injections for spinal stenosis. Pain. 159:876–83. DOI: 10.1097/j.pain.0000000000001158. PMID: 29394207.
9. Abdul AJ, Ghai B, Bansal D, Sachdeva N, Bhansali A, Dhatt SS. 2017; Hypothalamic pituitary adrenocortical axis suppression following a single epidural injection of methylprednisolone acetate. Pain Physician. 20:E991–1001. DOI: 10.36076/ppj/2017.7.E991. PMID: 29149147.
10. Iranmanesh A, Gullapalli D, Singh R, Veldhuis JD. 2017; Hypothalamo-pituitary-adrenal axis after a single epidural triamcinolone injection. Endocrine. 57:308–13. DOI: 10.1007/s12020-017-1357-7. PMID: 28674775. PMCID: PMC5554884.
11. Jayaraj VJ, Rampal S, Ng CW, Chong DWQ. 2021; The epidemiology of COVID-19 in Malaysia. Lancet Reg Health West Pac. 17:100295. DOI: 10.1016/j.lanwpc.2021.100295. PMID: 34704083. PMCID: PMC8529946.
12. Bugeja M, Mariani J, Dowling J, Stringaro G, Portelli JL, Sant K, et al. 2021; Musculoskeletal steroid injections during the COVID-19 pandemic. J Orthop. 26:103–6. DOI: 10.1016/j.jor.2021.07.017. PMID: 34312576. PMCID: PMC8294711.
13. McKean D, Chung SL, Fairhead R, Bannister O, Magliano M, Papanikitas J, et al. 2020; Corticosteroid injections during the COVID-19 pandemic: experience from a UK centre. Bone Jt Open. 1:605–11. DOI: 10.1302/2633-1462.19.BJO-2020-0130.R1. PMID: 33215158. PMCID: PMC7659632.
14. Azwan Aziz M, Abu Hanifah R, Mohd Nahar AM. 2021; Musculoskeletal corticosteroid injection during COVID-19 pandemic in Sabah: is it safe? Adv Orthop. 2021:8863210. DOI: 10.1155/2021/8863210. PMID: 33824767. PMCID: PMC8006753.
15. Chang CY, Prabhakar A, Staffa SJ, Husseini JS, Kheterpal AB, Simeone FJ, et al. 2021; Symptomatic COVID-19 infections in outpatient image-guided corticosteroid injection patients during the lockdown phase. Skeletal Radiol. 50:1117–23. DOI: 10.1007/s00256-020-03656-w. PMID: 33108512. PMCID: PMC7590247.
Fig. 1
Flow chart for enrollment, data collection, and data analysis. CPRC: Crisis Preparedness and Response Centre.
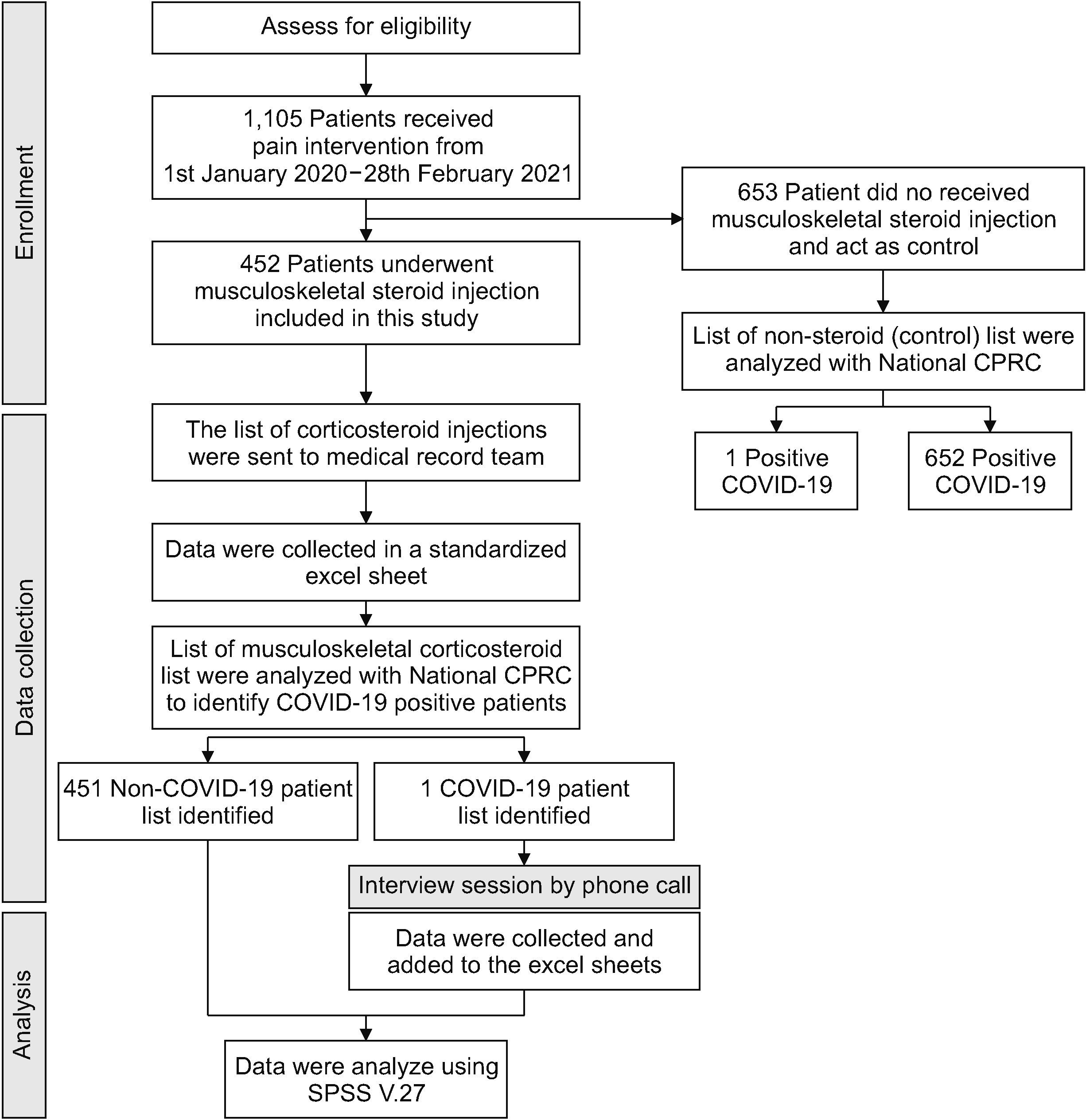
Table 1
Common region for musculoskeletal injections in sports medicine clinic, University Malaya Medical Centre
Table 2
Common diagnosis for musculoskeletal steroid injection in sports medicine clinic, University Malaya Medical Centre (n = 502)
Table 3
Risk analysis between steroid group and control group
Cross tabulation analysis were performed to look into the magnitude of the association, representing the relative risk (RR).
RR > 1.0 indicating the likelihood of contracting COVID-19 following musculoskeletal steroid injection.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download