1. Tatton-Brown K, Hanks S, Ruark E, Zachariou A, Duarte Sdel V, Ramsay E, et al. 2011; Germline mutations in the oncogene
EZH2 cause Weaver syndrome and increased human height. Oncotarget. 2:1127–33. DOI:
10.18632/oncotarget.385. PMID:
22190405. PMCID:
PMC3282071.
2. Tatton-Brown K, Murray A, Hanks S, Douglas J, Armstrong R, Banka S, et al. 2013; Weaver syndrome and
EZH2 mutations: Clarifying the clinical phenotype. Am J Med Genet A. 161A:2972–80. DOI:
10.1002/ajmg.a.36229. PMID:
24214728.
3. Byun JC, Kim CS, Lee SL, Kwon TC, Lee HJ. 2004; A case of Weaver syndrome. Korean J Pediatr. 47:1216–9.
4. Cohen AS, Yap DB, Lewis ME, Chijiwa C, Ramos-Arroyo MA, Tkachenko N, et al. 2016; Weaver syndrome-associated EZH2 protein variants show impaired histone methyltransferase function
in vitro. Hum Mutat. 37:301–7. DOI:
10.1002/humu.22946. PMID:
26694085. PMCID:
PMC4832389.
5. Polonis K, Blackburn PR, Urrutia RA, Lomberk GA, Kruisselbrink T, Cousin MA, et al. 2018; Co-occurrence of a maternally inherited
DNMT3A duplication and a paternally inherited pathogenic variant in
EZH2 in a child with growth retardation and severe short stature: atypical Weaver syndrome or evidence of a
DNMT3A dosage effect? Cold Spring Harb Mol Case Stud. 4:a002899. DOI:
10.1101/mcs.a002899. PMID:
29802153. PMCID:
PMC6071565.
6. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. 2015; Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 17:405–24. DOI:
10.1038/gim.2015.30. PMID:
25741868. PMCID:
PMC4544753.
7. Gan L, Yang Y, Li Q, Feng Y, Liu T, Guo W. 2018; Epigenetic regulation of cancer progression by
EZH2: from biological insights to therapeutic potential. Biomark Res. 6:10. DOI:
10.1186/s40364-018-0122-2. PMID:
29556394. PMCID:
PMC5845366.
9. Qiao Q, Li Y, Chen Z, Wang M, Reinberg D, Xu RM. 2011; The structure of
NSD1 reveals an autoregulatory mechanism underlying histone H3K36 methylation. J Biol Chem. 286:8361–8. DOI:
10.1074/jbc.M110.204115. PMID:
21196496. PMCID:
PMC3048720.
10. Tatton-Brown K, Rahman N. 2013; The NSD1 and
EZH2 overgrowth genes, similarities and differences. Am J Med Genet C Semin Med Genet. 163C:86–91. DOI:
10.1002/ajmg.c.31359. PMID:
23592277. PMCID:
PMC4845886.
11. Jeffries AR, Maroofian R, Salter CG, Chioza BA, Cross HE, Patton MA, et al. 2019; Growth disrupting mutations in epigenetic regulatory molecules are associated with abnormalities of epigenetic aging. Genome Res. 29:1057–66. DOI:
10.1101/gr.243584.118. PMID:
31160375. PMCID:
PMC6633263.
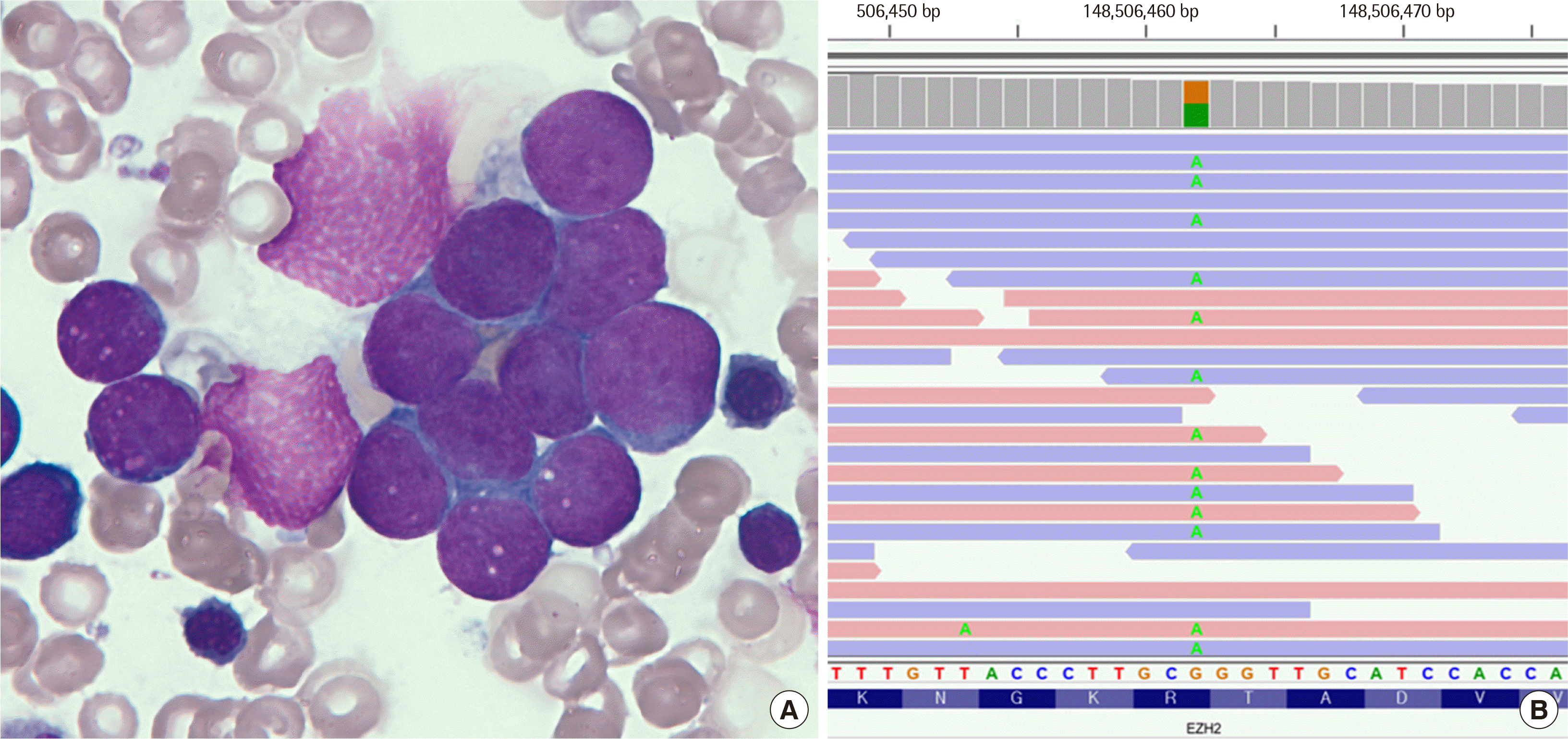
14. Usemann J, Ernst T, Schäfer V, Lehmberg K, Seeger K. 2016;
EZH2 mutation in an adolescent with Weaver syndrome developing acute myeloid leukemia and secondary hemophagocytic lymphohistiocytosis. Am J Med Genet A. 170A:1274–7. DOI:
10.1002/ajmg.a.37562. PMID:
26762561.
16. Kamien B, Ronan A, Poke G, Sinnerbrink I, Baynam G, Ward M, et al. 2018; A clinical review of generalized overgrowth syndromes in the era of massively parallel sequencing. Mol Syndromol. 9:70–82. DOI:
10.1159/000484532. PMID:
29593474. PMCID:
PMC5836217.
17. Mussa A, Duffy KA, Carli D, Griff JR, Fagiano R, Kupa J, et al. 2019; The effectiveness of Wilms tumor screening in Beckwith-Wiedemann spectrum. J Cancer Res Clin Oncol. 145:3115–23. DOI:
10.1007/s00432-019-03038-3. PMID:
31583434. PMCID:
PMC6876630.
18. Tatton-Brown K, Seal S, Ruark E, Harmer J, Ramsay E, Del Vecchio Duarte S, et al. 2014; Mutations in the DNA methyltransferase gene
DNMT3A cause an overgrowth syndrome with intellectual disability. Nat Genet. 46:385–8. DOI:
10.1038/ng.2917. PMID:
24614070. PMCID:
PMC3981653.
21. Villani A, Greer MC, Kalish JM, Nakagawara A, Nathanson KL, Pajtler KW, et al. 2017; Recommendations for cancer surveillance in individuals with RASopathies and other rare genetic conditions with increased cancer risk. Clin Cancer Res. 23:e83–90. DOI:
10.1158/1078-0432.CCR-17-0631. PMID:
28620009.
22. Al-Salem A, Alshammari MJ, Hassan H, Alazami AM, Alkuraya FS. 2013; Wea-ver syndrome and defective cortical development: a rare association. Am J Med Genet A. 161A:225–7. DOI:
10.1002/ajmg.a.35660. PMID:
23239504.
23. Turkkahraman D, Sakarya ANP, Randa NC. 2021; A novel
EZH2 gene variant in a case of Weaver syndrome with postaxial polydactyly. Am J Med Genet A. 185:2234–7. DOI:
10.1002/ajmg.a.62189. PMID:
33788986.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download