2. Other platelet function tests
Currently, there are several platelet function tests that can be utilized in clinical laboratories. Light transmission aggregometry (LTA) is the most widely used platelet function test [
14]. LTA uses platelet-rich plasma with various platelet agonists, such as epinephrine, adenosine diphosphate (ADP), collagen, arachinonic acid and ristocetin. It measures the change of optical density or turbidity which is induced by agonists in platelet-rich plasma [
15,
16]. An impaired or abnormal platelet aggregation pattern with more than one agonist suggests platelet function defects, and the type of abnormal aggregation pattern will guide further work-up process [
5,
16]. For example, an absence of aggregation to all agonists except ristocetin indicates Glanzmann thrombasthenia , absent response to ristocetin suggests Bernard–Soulier syndrome, and a markedly decreased or absent aggregation with ADP suggests P2Y12 receptor abnormality [
17]. Increased platelet aggregation to low concentrations of ristocetin is compatible with platelet-type von Willebrand disease (vWD) [
3,
14,
17]. LTA is considered as the gold standard for evaluating platelet function [
18]. However, it requires fresh blood, in relatively large volumes (20-50 mL), it is time-consuming, and it is a technically complicated test that requires experienced laboratory personnel [
9,
18,
19]. Consequently, LTA cannot be performed routinely in many laboratories. In addition, LTA results may be normal or only slightly defective in some mild platelet function defects; therefore, a normal LTA result by itself does not completely exclude a platelet function disorder [
14,
16,
20].
Whole blood aggregometry (WBA) is an alternative platelet aggregation test that measures the change in electrical resistance between two electrodes immersed in a whole blood sample, due to adhesion of platelets to the electrodes and platelet aggregation [
21]. Like LTA, WBA provides a detailed study of various platelet activation signaling pathways using multiple agonists with various concentrations [
3,
22]. It may be more sensitive to monitor antiplatelet therapy and some platelet function defects than LTA [
22]. In addition, WBA overcomes the sample volume issue of LTA, and does not require sample manipulation which can lead to potential delay of time and platelet activation during centrifugation [
3,
22]. Although the above-mentioned advantage of WBA in its simplified specimen handing process and technical demands, its reproducibility, sensitivity, and specificity are yet to be established in most platelet function disorders, in addition to poor correlation with LTA and a doubtful ability to predict clinical outcomes [
3,
14].
Viscoelastic tests, such as thromboelastography (TEG) or rotational thromboelastography (ROTEM) can monitor the rate and quality of clot formation using whole blood [
3]. It provides various profile of clot formation and clot strength resulting from interactions between platelets and coagulation factors [
3,
23]. Recently, the TEG
® PlateletMapping
® Assay using platelet agonists arachinonic acid and ADP has been introduced, and it can be used for monitoring of antiplatelet therapy [
3]. However, it measures clot properties only; therefore, the platelet function evaluation results are neither specific nor diagnostic [
3,
23].
The primary clinical utility of TEG and ROTEM tests is identifying hemostatic defects quickly in bleeding patients [
24]. These have been traditionally utilized in surgical and anesthesiologic departments as point-of-care tests for determining the risk of bleeding and as a guide to hemostatic therapy and transfusion requirements [
3,
24]. Although a TEG- and ROTEM-guided strategy can reduce the frequency of blood product transfusion compared to an empirical or central laboratory test-based transfusion strategy, it does not improve clinical outcomes and survival in various surgical patients [
3,
24].
The platelet function analyzer, INNOVANCE PFA-100/200
® system (Siemens Healthcare, Germany) has been widely used in many clinical laboratories [
3]. It is a cartridge-based point-of-care assay, in which whole blood is aspirated through a small aperture (150 µm in diameter) in a membrane coated with collagen and epinephrine or ADP [
3]. Under the high shear conditions, these platelet activators lead to platelet plug formation, and eventually occludes the aperture [
3]. The instrument records the time until the full occlusion of the aperture as “closure time” up to a maximum of 300 seconds [
3]. PFA-100/200 is simple, rapid, non-invasive, and only requires a small blood volume [
3]. Characteristically, the PFA 100/200 results are highly dependent on serum von Willebrand factor levels due to the high shear conditions within the cartridge; therefore, it can be a supplement test for the detection of vWD [
3,
25]. However, it should be noted that PFA-100/200 lacks a uniform high sensitivity and specificity for vWD, therefore, von Willebrand factor-specific testing should be performed if vWD is highly suspected [
26,
27].
There are various preanalytical and analytical variables that can affect the closure time. Among them, perhaps the most relevant variables are low platelet count (less than 50×109/L) or hematocrit levels (less than 25%); therefore, it is not a suitable test for patients with severe thrombocytopenia and suspected platelet function disorders [
3,
14,
25,
28]. In addition, some drugs, such as aspirin and non-steroidal anti-inflammatory drugs, can also affect the closure time [
25]. The interpretation of PFA-100/200 closure time according to the patterns and the degree of closure time prolongation with collagen-epinephrine (C/Epi) and collagen-ADP (C/ADP) cartridge is shown in
Fig. 1.
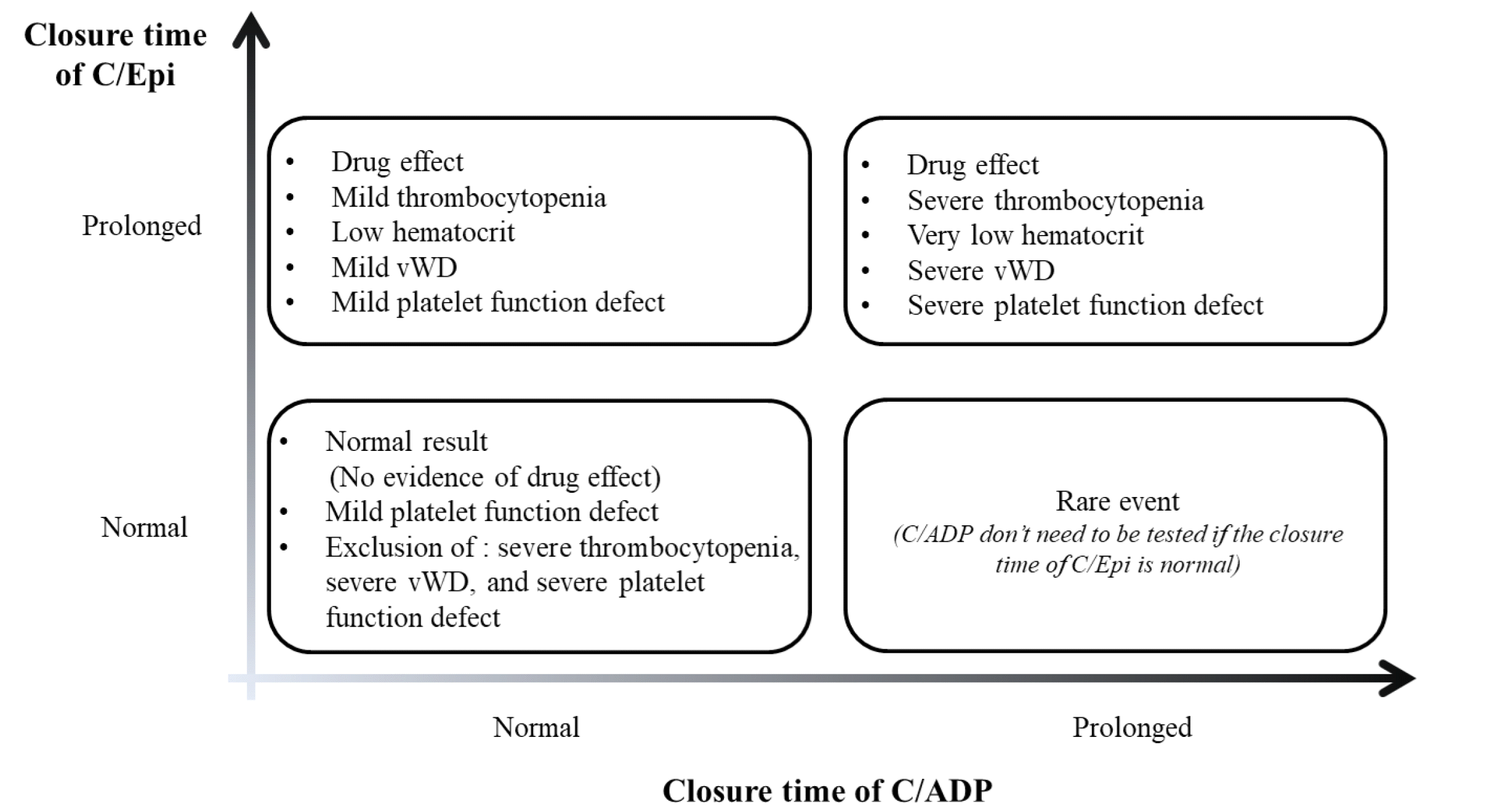 | Fig. 1A representative of clinically possible situations according to PFA-100/200 closure time with C/Epi and C/ADP cartridges (modified from [ 25]). Abbreviations: C/Epi, collagen-epinephrine; C/ADP, collagen-adenosine diphosphate; vWD, von Willebrand disease. 
|
Although the PFA-100/200 cannot be totally replaceable compared to BT, it can provide information on whether there is an abnormality in some platelet signaling pathways or not. Therefore, PFA-100/200 has been regarded as a practical and readily available platelet function screening test based on its advantages [
3,
25,
29]. Unfortunately, the closure time is insensitive to the detection of mild platelet function defects except for vWD. Mezzano et al. [
9] reported that the PFA-100/200 closure time was not significantly different between the patients with bleeding disorders and healthy control. The sensitivity of PFA-100/200 results for screening of bleeding disorders was approximately 30%, which is similar to that of BT [
9]. Interestingly, Podda et al. [
29] reported that the closure time of PFA-100/200 with C/Epi cartridge was significantly prolonged as the severity of the bleeding score increased, but not with C/ADP cartridge, which suggested a possibility to use C/Epi closure time results as a predictor of bleeding risk. However, in general, prolonged PFA-100/200 closure time does not necessarily imply increased hemorrhagic risk [
25]. Currently, the routine use of the PFA-100/200 for screening of abnormal platelet function is not recommended due to the lack of reproducibility, and low sensitivity and specificity for platelet function defects [
5,
14]. In addition, the prolonged closure time cannot distinguish vWD from other platelet function diseases, and a normal closure time does not rule out mild platelet function disorders [
14]; therefore, it is an “optional” screening test for platelet function diseases [
6,
25]. If the clinical suspicion of platelet function defects is high, normal PFA-100/200 results should not rule out the possibility of platelet function defects; therefore, specific platelet function assay is indicated [
6,
14,
25]. Patients with sufficient clinical suspicion of mild or moderate platelet function disorders should undergo further diagnostic testing irrespective of PFA-100/200 results [
25].
PFA-100/200 has little value for assessing surgical bleeding risk in presurgical patients without formal preoperative assessment of medication, personal, and family history [
12,
25]. However, the utility of closure times depend on the patient population under investigation [
25]. For example, if a patient has a bleeding history, PFA-100/200 is held as worthwhile in screening whether the abnormal bleeding is associated with platelet dysfunction [
12].





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download