1. Ruskova A, Thula R, Chan G. 2004; Aggressive natural killer-cell leukemia: report of five cases and review of the literature. Leuk Lymphoma. 45:2427–38. DOI:
10.1080/10428190400004513. PMID:
15621755.
2. Park S, Ko YH. 2014; Epstein-Barr virus-associated T/natural killer-cell lymphoproliferative disorders. J Dermatol. 41:29–39. DOI:
10.1111/1346-8138.12322. PMID:
24438142.
3. Ryder J, Wang X, Bao L, Gross SA, Hua F, Irons RD. 2007; Aggressive natural killer cell leukemia: report of a Chinese series and review of the literature. Int J Hematol. 85:18–25. DOI:
10.1532/IJH97.A10612. PMID:
17261497.
5. Hutchens C, Ketterling RP, Van Dyke DL. 2012; When are apparently non-clonal abnormalities in bone marrow chromosome studies actually clonal? Cancer Genet. 205:405–9. DOI:
10.1016/j.cancergen.2012.04.003. PMID:
22868001.
6. Suzuki R, Suzumiya J, Nakamura S, Aoki S, Notoya A, Ozaki S, et al. 2004; Aggressive natural killer-cell leukemia revisited: large granular lymphocyte leukemia of cytotoxic NK cells. Leukemia. 18:763–70. DOI:
10.1038/sj.leu.2403262. PMID:
14961041.
7. Heyer EE, Deveson IW, Wooi D, Selinger CI, Lyons RJ, Hayes VM, et al. 2019; Diagnosis of fusion genes using targeted RNA sequencing. Nat Commun. 10:1388. DOI:
10.1038/s41467-019-09374-9. PMID:
30918253. PMCID:
PMC6437215.
8. Singh S, Qin F, Kumar S, Elfman J, Lin E, Pham LP, et al. 2020; The landscape of chimeric RNAs in non-diseased tissues and cells. Nucleic Acids Res. 48:1764–78. DOI:
10.1093/nar/gkz1223. PMID:
31965184. PMCID:
PMC7038929.
9. Biernaux C, Loos M, Sels A, Huez G, Stryckmans P. 1995; Detection of major bcr-abl gene expression at a very low level in blood cells of some healthy individuals. Blood. 86:3118–22. DOI:
10.1182/blood.V86.8.3118.3118. PMID:
7579406.
10. Bose S, Deininger M, Gora-Tybor J, Goldman JM, Melo JV. 1998; The presence of typical and atypical BCR-ABL fusion genes in leukocytes of normal individuals: biologic significance and implications for the assessment of minimal residual disease. Blood. 92:3362–7. DOI:
10.1182/blood.V92.9.3362. PMID:
9787174.
11. Oh S, Shao J, Mitra J, Xiong F, D'Antonio M, Wang R, et al. 2021; Enhancer release and retargeting activates disease-susceptibility genes. Nature. 595:735–40. DOI:
10.1038/s41586-021-03577-1. PMID:
34040254.
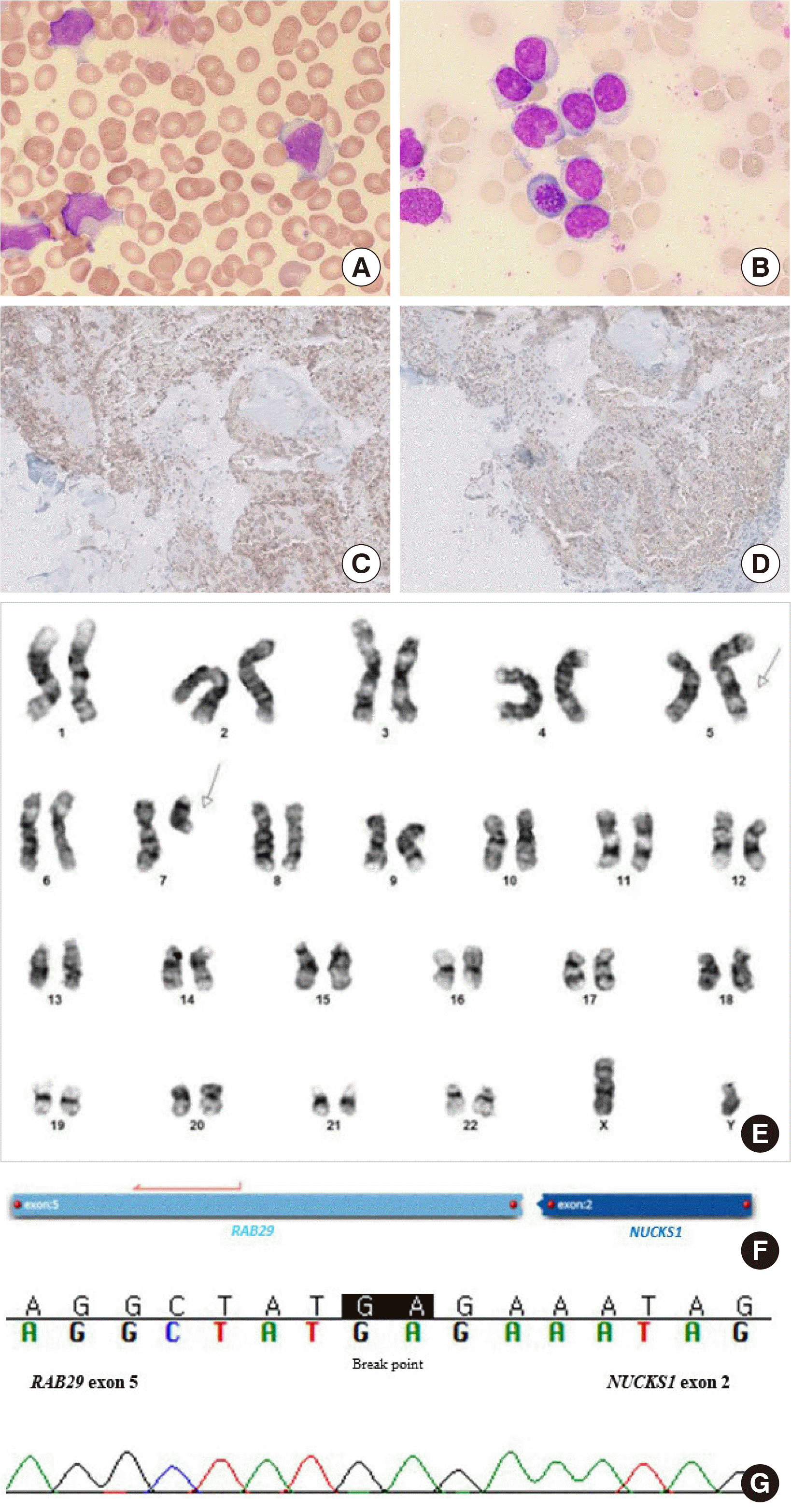
13. Wang S, Ma Z, Xu X, Wang Z, Sun L, Zhou Y, et al. 2014; A role of Rab29 in the integrity of the trans-Golgi network and retrograde trafficking of mannose-6-phosphate receptor. PLoS One. 9:e96242. DOI:
10.1371/journal.pone.0096242. PMID:
24788816. PMCID:
PMC4008501.
14. Gary-Bobo M, Nirdé P, Jeanjean A, Morère A, Garcia M. 2007; Mannose 6-pho-sphate receptor targeting and its applications in human diseases. Curr Med Chem. 14:2945–53. DOI:
10.2174/092986707782794005. PMID:
18220730. PMCID:
PMC2793280.
15. Abdoli Z, Assarehzadegan MA, Pipelzadeh MH, Iranparast S, Dashti Gerdabi N, Parsanahad M, et al. 2021; Leukemia inhibitory factor suppresses NKG2D mRNA expression and presentation on human natural killer cells. Iran J Allergy Asthma Immunol. 20:98–105. DOI:
10.18502/ijaai.v20i1.5416. PMID:
33639636.
16. Parplys AC, Zhao W, Sharma N, Groesser T, Liang F, Maranon DG, et al. 2015; NUCKS1 is a novel RAD51AP1 paralog important for homologous recombination and genome stability. Nucleic Acids Res. 43:9817–34. DOI:
10.1093/nar/gkv859. PMID:
26323318. PMCID:
PMC4787752.
17. Huang P, Cai Y, Zhao B, Cui L. 2018; Roles of NUCKS1 in diseases: Susceptibility, potential biomarker, and regulatory mechanisms. Biomed Res Int. 2018:7969068. DOI:
10.1155/2018/7969068. PMID:
29619377. PMCID:
PMC5830027.
18. Frattini V, Trifonov V, Chan JM, Castano A, Lia M, Abate F, et al. 2013; The integrated landscape of driver genomic alterations in glioblastoma. Nat Genet. 45:1141–9. DOI:
10.1038/ng.2734. PMID:
23917401. PMCID:
PMC3799953.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download