Abstract
The author experienced a case of autologous platelet-rich plasma (PRP) affecting the recovery of a chronic neuropathic diabetic foot ulcer combined with infection. A 65-year-aged male with uncontrolled diabetes presented with a Wagner grade 2 diabetic foot ulcer on his left forefoot of more than 2 weeks duration. Osteomyelitis, gangrene, and ischemia requiring acute intervention were absent. Although infection was controlled to a moderate degree, wound healing was unsatisfactory following surgical debridement and simple dressing. Therefore, intralesional autologous PRP injection was performed 5 times as an adjuvant regeneration therapy, and the recalcitrant ulcer healed in 3 months. Intralesional PRP injections are worthwhile as they promote wound regeneration, are evidence-based, safe, and can be easily performed in ambulatory care facilities.
Wound healing is impaired in diabetes, because the biochemical and immunological pathologies up to cellular level affect the entire process of wound recovery.1) In diabetic foot, wound healing is 2 to 3 times slower than non-diabetes.2) Chronic nonhealing foot ulcers occur in approximately 15% of patients with diabetes.1) Because chronicity in diabetic foot ulcer (DFU) significantly increases healthcare costs and the possibility of infection and lower leg amputation, early regeneration treatment with infection control and blood flow improvement are important for the prognosis. Tissue regeneration therapies using living cells and biosynthetic materials have become available recently. However, most of these are still expensive or not readily applicable for many reasons.1) Because the regeneration and immune modulation effects of platelet-rich plasma (PRP) on DFU have been sufficiently proven, the author planned intralesional autologous PRP injection for a chronicized recalcitrant Wagner grade 2 DFU.3-5)
This manuscript was exempted from deliberation by the Institutional Review Board as a case report, and informed consent for publication of the clinical data was obtained from the study participant.
On February 8, 2022, a 65-year-aged male with uncontrolled diabetes visited our clinic with a DFU on the medial side of his left forefoot. The ulcer, had passed more than 2 weeks from onset, was in a chronic state when he arrived. The surface of the ulcer was dried, and there was necrotic tissue with full-thickness subcutaneous loss in the central area. It was consistent with Wagner grade 2 combined with cellulites/erythema extending >2 cm around the ulcer border. The infection state was moderate degree according to the IDSA/IWGDF classification.6) There was a subcutaneous abscess with purulent discharge in the inflammation area which became ulcerous change after 2 days. It was also thought to neuropathic ulcer because the sensation of the foot and ulcer area were dull, with the feeling of a callus on the sole of the foot, and there was little pain compared to the state of inflammation. No signs of systemic infection, osteomyelitis, and ischemia, which require hospitalization and acute intervention, were observed (Fig. 1).
The patient had developed blisters after climbing more than 14 days previous and had treated himself for about a week. As the lesion worsened, he was referred to a tertiary hospital 7 days prior to visiting the clinic. But, after a blood flow test was performed 2 days previous, he refused more workup and treatment. He said he heard that there were no abnormalities in the blood flow test conducted at the tertiary hospital. On his first visit, he was taking 500 mg/day of metformin, and the blood test results of erythrocyte sedimentation rate, glucose (Fasting, Serum), and HbA1c were 74, 140 mg/dL, and 9.7%, respectively. There was no fever and other signs of major illness. He refused to be referred to a tertiary hospital for skin graft or flap surgery, and only wanted private office care for personal reasons. The author planed diabetes control through serum glucose and HbA1C test monitoring, and wound care with local infection control. Intravenous cefazolin was administered for 10 days and oral cefadroxil was used until the signs of local infection disappeared as empirical antibiotics. Surgical debridement and wound dressing were performed at every visit. Each time of debridement, all the obvious necrotic tissue, and cellular burden of dead and senescent cells were removed. At each dressing, potadine was completely cleaned with sterile normal saline, and antibiotic ointment with gauze dressing was applied. After infection controlled, hydrocolloid bandage was attached during daytime, and at night, ointment containing epidermal growth factor was applied. Every visiting day, supportive percutaneous electro-magnetic therapy (TESLA-3000; Wever Instruments, Uijeongbu, Korea) on the involved foot for nerve stimulation and circulation improvement, although randomized controlled trials in patients with DFUs have not demonstrated benefits.5) Other than the treatment described above, treatment methods such as hyperbaric oxygen therapy which has detrimental effect on bacteria and negative-pressure wound therapy were not applied. Although signs of local infection around the ulcer disappeared within 10 days, tissue regeneration was too slow, and it was expected that full tissue recovery would be difficult. Therefore, we performed intralesional injection of autologous PRP 5 times as adjuvant regeneration therapy with irregularly through out-patient care. After the 3rd PRP injection, margin in-growth of the ulcer bed, granulation tissue development, vascularization and re-epithelialization and the sense of pain improved more rapidly, although the deepest ulcer area, which reached near fascia, hardly regenerated (Fig. 2).
For the autologous PRP procedure, 27 mL of venous blood was collected in a 30 mL syringe containing 3 mL of ACD-A solution (NOTHROM Soln.; DAE HWA Pharm. Co., Ltd., Seoul, Korea). The PRP was separated by centrifuging at 3200 RPM for 5 minutes (Fleta 40P; Hanil Scientific Inc., Gimpo, Korea) using a TriCell (REV-MED Inc., Seongnam, Korea) device. Three to four milliliters of leukocyte- and platelet-rich plasma (L-PRP) was injected every time. Following the 5th PRP injection, the chronicized DFU recovered within 3 months with no defect (Fig. 3, 4). PRP gel with hydrogel dressing was applied to the superficial wounds of the opposite toes, which healed in 3 weeks (Fig. 3). To acquire the PRP gel, calcium gluconate (9.3 mg/mL) and patient’s serum, as autologous thrombin, were used.7)
A chronicized Wagner grade 2 forefoot located DFU (area ≥1 cm2, loss of protective sensation and presence of bacterial infection with no peripheral arterial disease or osteomyelitis) was successfully healed by applying autologous PRP in addition to general basic wound care. Healing time (i.e., from the date of the patient’s first visit to the clinic to the date at which the DFU had epithelialized and was deemed healed by the clinician) was about 81 days. It took about 74 days for the patient to recover from the date of the first surgical debridement. This is 2 or 3 times longer than a non-diabetic healing period, but it is similar to or better than results in multidisciplinary treatment data. Milne et al.2) reported 83 days (mean 122 days) for DFU with soft tissue infection only. In addition, they reported that 8% of unhealed. Zimny et al.8) reported that the wound radius decreased by 0.045 mm (95% confidence interval [CI], 0.039∼0.055 mm) per day and the average healing time was 77.7 days (95% CI, 62∼93 days) in neuropathic DFU with no ischemic or peripheral occlusive vascular diseases.
Between 19% and 34% of diabetes patients are affected DFU during their lifetime, and the rate of recurrence of DFU is more than 50% after 3 years.8) According to a retrospective study by Monami et al.,9) 53.8% of 196 DFU patients did not heal within 6 months, and a conservative approach was found to be more economical and convenient than major amputation as treatment. Diabetic foot problems impose a major economic burden, and costs increase proportionately to the severity of the condition. Therefore, from this point of view, wound recovery in the early stage is very important when DFU first occur.
In the Rep. of Korea, the Wagner system is the one most frequently used for DFU classification.10) Wagner grade 3 (deep abscess formation or osteomyelitis) or higher (presence of gangrene) are major predictors of amputation in diabetic foot infection. The risk of amputation increases when local findings such as ulcers reaching bone, hindfoot position, decreased ankle-brachial index, and peripheral arterial disease are observed. Therefore, close monitoring and transfer to multidisciplinary care must be considered when the above local findings are noted.
In diabetes, all phases of wound healing are affected. Angiogenesis and the migration of cells for tissue regeneration are diminished, causing deficient re-epithelialization. In the same way, the poor production of the extracellular matrix by fibroblasts contributes to the problem of deficient wound closure.4) PRP contains highly concentrated platelets, and other elements such as soluble proteins, hormones, and cell adhesion molecules. L-PRP contains neutrophils, monocytes, endothelial cells, keratinocytes and possibly mesenchymal stem cells. In addition to the functions of coagulation and immune modulation, platelets can regulate fundamental mechanisms in all wound healing phases including migration, proliferation and differentiation of dermal fibroblasts, and angiogenesis through the secretion of various growth factors such as epidermal growth factor (EGF), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF), transforming growth factor beta (TGF-β), and vascular endothelial growth factor (VEGF).1,4) Platelets also promote the tissue remodeling of aged skin by increasing type I collagen and matrix metalloproteinases gene expression, and the recruitment, adhesion, and proliferation of adult stem cells. In particular, platelet-derived extracellular vesicles carry various cargo including lipids, anchored membrane receptors, cytosolic proteins such as growth factors, proteins transcription factors and RNA molecules. A recent study revealed that new proteins can be synthesized by platelets and platelets can actively transfer RNAs to other cells. Among the RNAs, messenger RNAs incorporated into the target cells can be translated into proteins and micro RNAs have been found to regulate gene expression, resulting in functional changes in the target cells.7) Therefore, especially in the case of diabetic foot where blood flow is hindered by vasculopathies, and neuropathy caused by diabetes, external supply of PRP can help wound healing. The injection of L-PRP into the inflammatory lesion was thought to be helpful for immune modulation and infection control.
IWGDF basically recommended the followings as local ulcer care; regular inspection of the ulcer, debridement, appropriate dressing, do not soaking and considering negative pressure for postoperative wound. In addition, IWGDF recommends following several adjunctive treatments can be considered in noninfected ulcers that fail to heal after 4 to 6 weeks despite optimal clinical care; sucrose octasulfate impregnated dressing, placental membrane allograft, systemic oxygen therapy as an adjuvant treatment in ischemic ulcers do not heal despite revascularization, and multilayered patches of autologous leucocytes, platelets, and fibrin can be considered for ulcers with or without moderate ischemia for routine ulcer management.3) Autologous L-PRP used in this case can be seen as a direct injection of the above patch recommended by IWGDF. In contrast to this, as for the routine ulcer management, IWGDF also does not recommend biological active product (collagen, growth factor, or bioengineered tissue) in neuropathic ulcers and silver/antimicrobial agent containing dressings or topical applications.
There are a wide range of agents available or currently being studied as adjuvant therapies including nonsurgical debridement agents, dressings and topical products, oxygen therapies, negative pressure wound therapy, acellular bioproducts, human growth factors, skin grafts and bioengineered skin, energy-based therapies, and systemic therapies. But there is a need for well-designed blinded random controlled trials to determine the true efficacy of these interventions and to develop evidence-based practice guidelines. Until then, good clinical judgment—considering the patient’s clinical context and wound characteristics—is essential to assess the risk and benefits of these adjuvant interventions for current clinical use.5) With technological advancement, engineered biological tissue products and living tissues materials have been developed. Although they are supported by clinical trials and represent potential new therapies for the future, they are still expensive and are less accessible.1) Autologous PRP injection is an evidence based, safe and cost-effective supportive therapy for every stage of DFU which require conservative care. They provide good accessibility to both clinicians and patients due to easy manipulation and immediate use. If adequate empirical antibiotics are used and the DFU are not accompanied by conditions which need require acute intervention, Wagner grade 1 and 2 DFU can be successfully treated without patient loss while providing convenience to patients even in ambulatory care facilities.
ACKNOWLEDGEMENTS
The author thanks Young-Jin Lee. M.D. for his endocrinological and neurological support.
REFERENCES
1. Andrews KL, Houdek MT, Kiemele LJ. 2015; Wound management of chronic diabetic foot ulcers: from the basics to regenerative medicine. Prosthet Orthot Int. 39:29–39. doi: 10.1177/0309364614534296. DOI: 10.1177/0309364614534296. PMID: 25614499.
2. Milne TE, Schoen DE, Bower VM, Burrows SA, Westphal C, Gurr JM. 2013; Healing times of diabetic foot ulcers: investigating the influence of infection and peripheral arterial disease. J Diabet Foot Complicat. 5:29–38.
3. Schaper NC, van Netten JJ, Apelqvist J, Bus SA, Hinchliffe RJ, Lipsky BA. 2020; Practical guidelines on the prevention and management of diabetic foot disease (IWGDF 2019 update). Diabetes Metab Res Rev. 36 Suppl 1:e3266. doi: 10.1002/dmrr.3266. DOI: 10.1002/dmrr.3266. PMCID: PMC7154668.
4. Scimeca CL, Bharara M, Fisher TK, Kimbriel H, Armstrong DG. 2010; Novel use of platelet-rich plasma to augment curative diabetic foot surgery. J Diabetes Sci Technol. 4:1121–6. doi: 10.1177/193229681000400510. DOI: 10.1177/193229681000400510. PMID: 20920431. PMCID: PMC2956802.
5. Everett E, Mathioudakis N. 2018; Update on management of diabetic foot ulcers. Ann N Y Acad Sci. 1411:153–65. doi: 10.1111/nyas.13569. DOI: 10.1111/nyas.13569. PMID: 29377202. PMCID: PMC5793889.
6. Lipsky BA, Senneville É, Abbas ZG, Aragón-Sánchez J, Diggle M, Embil JM, et al. International Working Group on the Diabetic Foot (IWGDF). 2020; Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. 36 Suppl 1:e3280. doi: 10.1002/dmrr.3280. DOI: 10.1002/dmrr.3280. PMCID: PMC7154668.
7. Kim MH, Byeon HS. 2019; Review for good platelet-rich plasma procedure in cosmetic dermatology and surgery. J Cosmet Med. 3:1–13. doi: 10.25056/JCM.2019.3.1.1. DOI: 10.25056/JCM.2019.3.1.1.
8. Zimny S, Schatz H, Pfohl M. 2002; Determinants and estimation of healing times in diabetic foot ulcers. J Diabetes Complications. 16:327–32. doi: 10.1016/s1056-8727(01)00217-3. DOI: 10.1016/S1056-8727(01)00217-3. PMID: 12200075.
9. Monami M, Ragghianti B, Nreu B, Lorenzoni V, Pozzan M, Silverii A, et al. Major amputation in non-healing ulcers: outcomes and economic issues. Data from a cohort of patients with diabetic foot ulcers. Int J Low Extrem Wounds. Published online April 28, 2022; doi: 10.1177/15347346221097283. DOI: 10.1177/15347346221097283. PMID: 35477285.
10. Won SH, Min TH, Chun DI, Bae SY. The Academic Committee of Korean Foot and Ankle Society. 2022; Current trends in the treatment of diabetic foot: analysis of the Korean Foot and Ankle Society (KFAS) member survey. J Korean Foot Ankle Soc. 26:30–9. doi: 10.14193/jkfas.2022.26.1.30. DOI: 10.14193/jkfas.2022.26.1.30.
Figure 1
Local appearances of a diabetic foot ulcer. (A) First visit. (B) Two days after the first visit. The subcutaneous abscess with purulent discharge in the inflammation area became to conglomerated to ulcer.
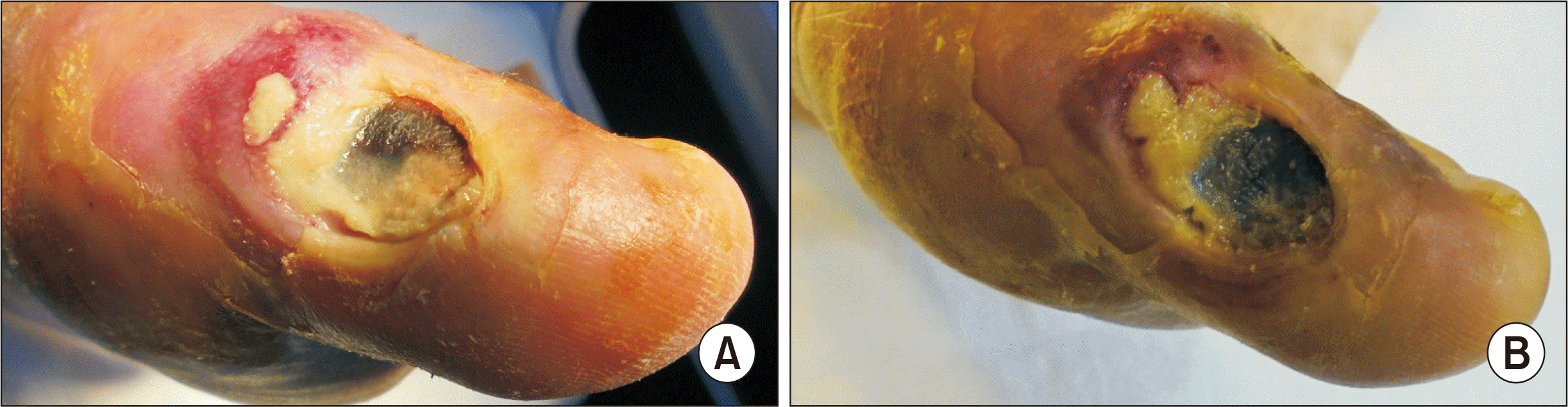
Figure 2
Change of the diabetic foot ulcer after intralesional autologous platelet-rich plasma (PRP) injection. (A) Five days after the 2nd PRP injection. (B) Twelve days after the 4th PRP injection. (C) Thirteen days after the 4th PRP injection.
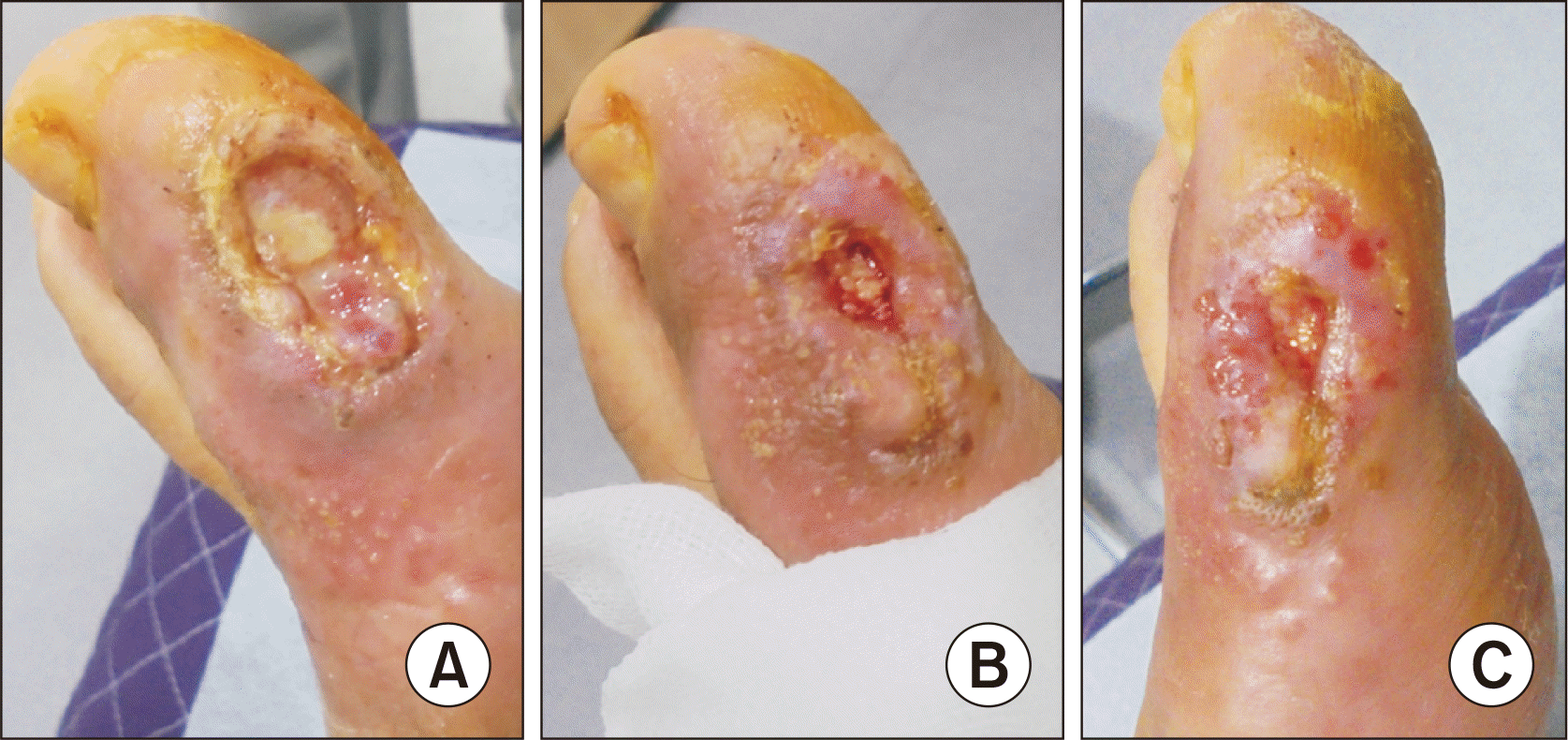
Figure 3
Photographs of procedure. (A, B) Fifth platelet-rich plasma (PRP) intralesional injection into the Wagner grade 2 diabetic foot ulcer on the left forefoot. (C, D) PRP gel was applied to the superficial wounds on the opposite toes.
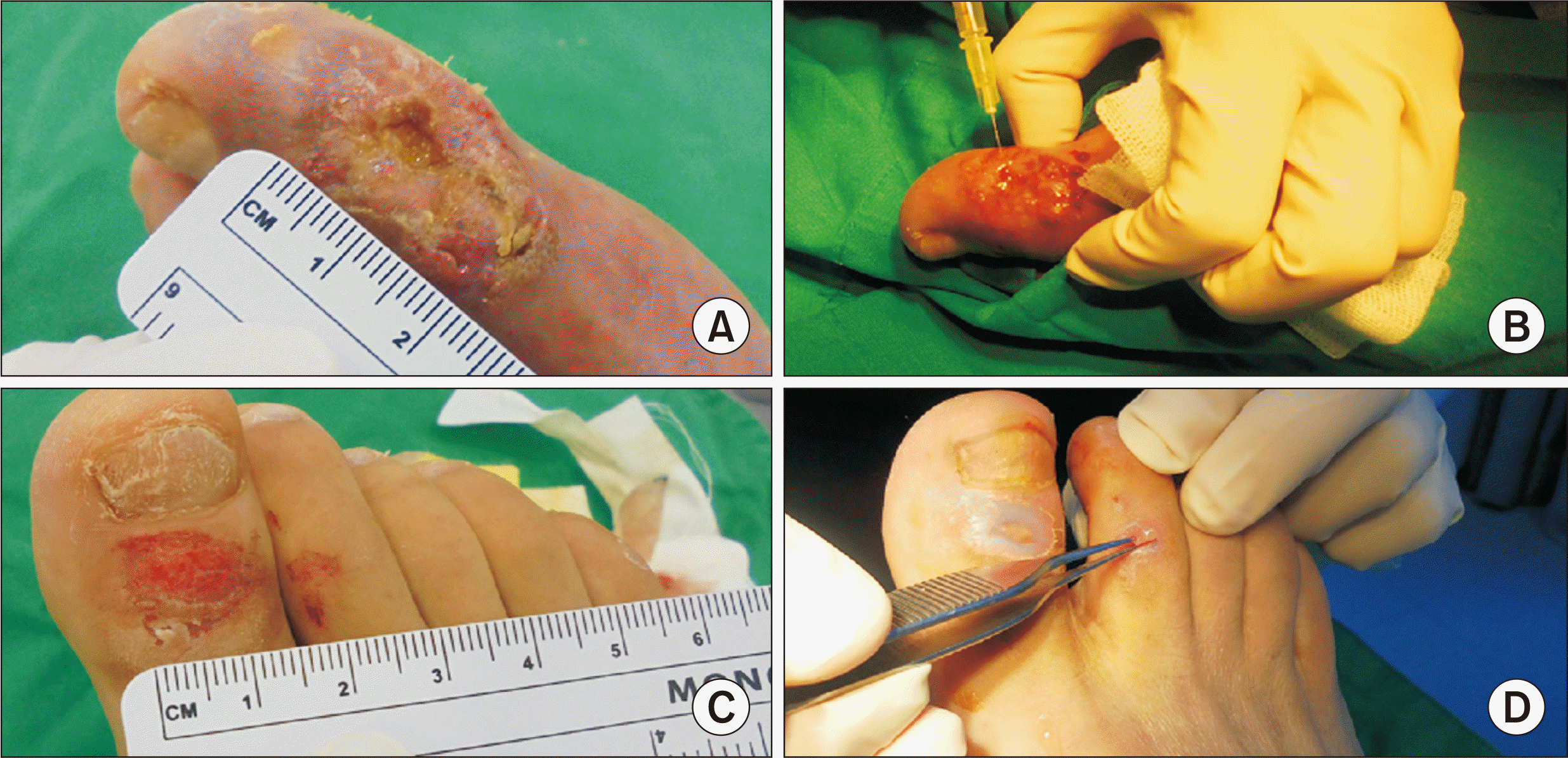
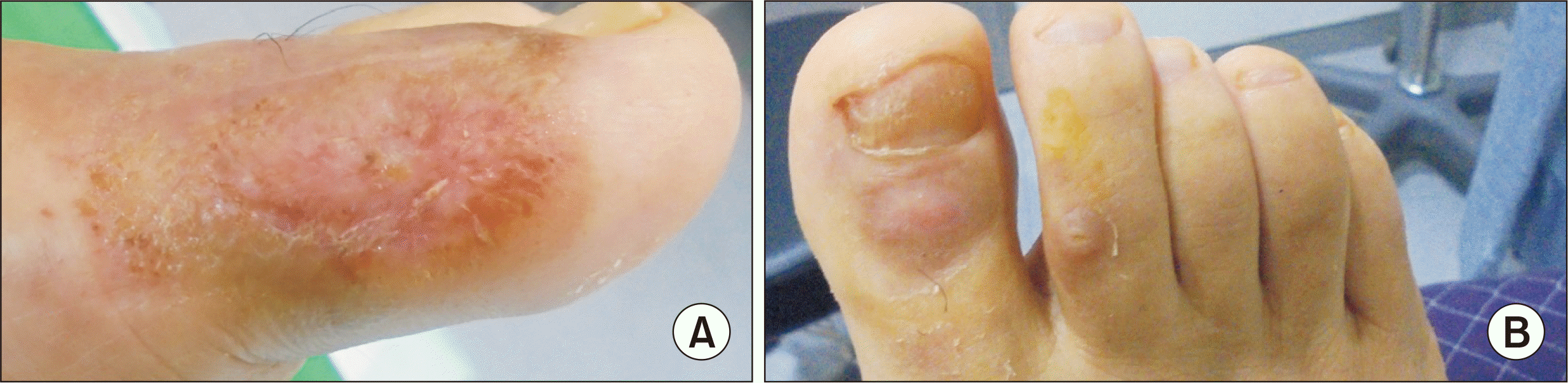
Figure 4
Photographs taken when the patient visit the clinic 6 weeks after the 5th platelet-rich plasma (PRP) injection. (A) Within 3 months from the first visit, the chronicized Wagner grade 2 diabetic foot ulcer was healed completely with no defect. (B) Superficial wounds on the opposite toes were healed in 3 weeks after PRP gel with hydrocolloid bandage application.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download