TO THE EDITOR: Serum protein electrophoresis (sPEP) and serum immunofixation electrophoresis (sIFE) are the gold standards for diagnosing monoclonal gammopathies (MGs) [1]. However, the PEP and IFE results may vary depending on the method, expertise of the laboratory personnel, and differences between laboratories [2]. Urine protein electrophoresis (uPEP) and urine IFE (uIFE) can be used with high sensitivity [3]; however, the results can be affected by renal function [4]. The free light chain (FLC) assay is an alternative method for diagnosing and monitoring MGs, and has several advantages [3]. First, owing to its short half-life, the FLC assay can be used for real-time monitoring of disease progression or response to treatment in patients with MG [5, 6]. Second, the FLC assay is more sensitive than the PEP and IFE [7]. They are immensely useful, especially in the follow-up of patients with low levels of monoclonal proteins, which account for 20% of MGs [5]. The analytical performance and clinical usefulness of serum FLC (sFLC) assays have been evaluated and compared in previous studies. However, data on urinary FLC (uFLC) assays are limited [8]. Therefore, this study aimed to evaluate and compare the usefulness of sFLC and uFLC assays for diagnosing MGs and other related diseases, and to determine their application in clinical practice.
From June to November 2021, the remaining pairs of serum and 24-h urine samples were collected from patients whose samples were submitted for sPEP, sIFE, uPEP, and uIFE tests as routine examinations. Serum samples were stored at -70°C, thawed, and assayed on the same day. However, urine samples were assayed on the day of collection to prevent the degeneration of urine proteins. We retrospectively reviewed patients' electronic medical records and collected the following data: age, sex, clinical diagnosis, whether the sample was collected at initial diagnosis or follow-up, response to treatment, bone marrow study results (if available), and estimated glomerular filtration rate (eGFR). The eGFR value was calculated using the Modification of Diet in Renal Disease 4-variable formula (isotope dilution mass spectrometry traceable), whereas the body surface area was calculated using the Dubois formula.
Protein electrophoresis and immunofixation were performed using the Sebia Capillarys 2 Flex Piercing System (Sebia, Lisses, France) using the following reagents: Capillarys Protein (E) 6 Kit for sPEP, Capillarys/Minicap Urine Kit for uPEP, and Capillarys Immunotyping Kit for sIFE and uIFE. The detection limit was 0.1 g/dL for sPEP and 2.0 mg/dL for uPEP. sPEP and uPEP results were considered positive if the levels of monoclonal proteins detectable by laboratory personnel were above the detection limit. Total protein and creatinine levels in serum were determined using a colorimetric method, and serum immunoglobulin (sIg) heavy chain and urinary total protein levels were determined using an immuno-turbidimetric method (Cobas c 702 module, Roche Diagnostics, Switzerland). sFLC and uFLC levels were measured using the Freelite assay (The Binding Site Group Ltd, Birmingham, UK), a latex-enhanced immunonephelometric assay measuring free k and l light chains, on an automated Cobas 8000 platform (Roche). The sensitivity of this FLC assay has been reported to be <1 mg/L [9]. The reference interval of FLCs established by the manufacturers was as follows: 3.3–19.4 mg/L for serum k, 5.7–26.3 mg/L for serum l, 0.26–1.65 for the serum k/l ratio, <32.70 mg/L for urine k, <4.99 mg/L for urine l, and 2.04–17.78 for the urine k/l ratio.
This study was approved by the Institutional Review Board of Wonju Severance Christian Hospital (IRB No. CR321321), which waived the requirement for informed consent.
The quantitative values of sFLC and uFLC were compared, and the sFLC and uFLC levels were compared according to the presence of MG [additionally subdivided into newly diagnosed/refractory or relapsed (ND/RR) or non-MD/RR]. In patients without MG, the sFLC and uFLC levels were compared according to the degree of renal insufficiency. Additionally, the clinical utility of sPEP/sIFE, uPEP/uIFE, sFLC, uFLC, and sIg for diagnosing MGs was evaluated using receiver operating characteristic (ROC) curve analysis, and the area under the curve (AUC) was compared. The agreement between each method and the sPEP was evaluated. The diagnostic performance was also compared, and the combination of each test method was used to determine the combination with the highest sensitivity and specificity.
Statistical analyses were performed using Statistical Package for the Social Sciences (SPSS, version 25.0, IBM Corp., Armonk, NY, USA) and Microsoft Excel 2019 (Microsoft Corp., Redmond, Washington, USA) with Analyse-it version 5.81 (Analyse-it Software, Ltd., Leeds, UK). Statistical significance was set at P<0.05.
Among the 123 study patients, 63 (51.2%) were male, and 60 (48.8%) were female. The median age of all patients was 66.5 years (IQR, 60.0–77.8 yr). Fifty-one (41.5%) patients were diagnosed with MG, of which 41 were considered to have ND/RR MG (Supplementary Table 1). The correlation between serum and urine FLCs was 0.576 for k, 0.615 for l, and 0.528 for the k/l ratio (Supplementary Fig. 1). The sFLC and uFLC levels were compared among patients with ND/RR MG, those with non-ND/RR MG, and those without MG (Table 1). The sFLC and uFLC levels of patients without MG were compared according to the degree of renal impairment by eGFR <30, 30–59, and <60 mL/min/1.73 m2 (Table 2).
The ROC curves of sPEP/sIFE, uPEP/uIFE, sFLC, uFLC, and sIgs for the diagnostic agreement for ND/RR MG were compared (Fig. 1). sPEP/sIFE showed a statistically significant difference compared to uPEP/uIFE (P=0.0301) but was not significantly different from sFLC (P=0.0733). The diagnostic performance of sFLC was significantly higher than that of uFLC (P<0.001). The agreement between each method compared with sPEP/sIFE and Cohen's kappa coefficients of uPEP/uIFE, sFLC, uFLC, and sIg were 0.692, 0.700, 0.484, and 0.377, respectively. Table 3 presents a comparison of the analytical performances of each method. Among these, sPEP/sIFE showed high sensitivity (92.7%) and specificity (96.3%). uPEP/uIFE and sFLC showed high specificities of 97.6% and 95.1%, respectively, but relatively low sensitivities of 75.6% and 80.5%. When the test methods were combined, the combination of sPEP/sIFE and uPEP/uIFE showed the highest sensitivity of 97.6%, whereas the combination of sPEP/sIFE and sFLC showed a sensitivity of 95.1%.
This study aimed to compare the analytical and diagnostic performance of sFLC and uFLC with sPEP/sIFE and uPEP/uIFE, and to unveil the role of the FLC assay in diagnosing MG and other diseases. Between the serum and urine samples, k and l FLC and the k/l ratio showed a good correlation, which was comparable to a previous study [6]. FLC levels were significantly higher in patients with ND/RR-MG. When patients without MG were divided according to the degree of renal insufficiency, the subgroup with the lower eGFR had higher serum k- and l-FLC, and the k/l ratio was statistically significant. Under healthy conditions, only a small amount of FLC is excreted in the urine because FLC is degraded rapidly by renal tubular reabsorption and rapidly metabolized in the proximal tubule. Therefore, the results of this study are consistent with the fact that a decrease in eGFR is associated with an increase in sFLC levels [10].
In the ROC analysis, sPEP/sIFE showed the highest AUC value, whereas sFLC showed a similar AUC value to uPEP/uIFE, and uFLC and sIg showed low AUC values. In this study, the sensitivity for diagnosing MG was 92.7% for sPEP/sIFE, and 97.6% for the combination of sPEP/sIFE and uPEP/uIFE. This result is similar to that of a previous study that reported sensitivities of 94.3% and 97.0%, respectively [11]. The diagnostic performance of sFLC and uPEP/uIFE was similar. Studies have been performed to evaluate if the sFLC assay could replace the uPEP/uIFE test [12]. Although complete replacement is still difficult owing to some discrepancies, these tests are complementary to each other, and more attention is required [1, 13].
This study has some limitations. First, the sample size is small. The reliability of the comparative results of assay performance can be improved by large-scale studies with a larger number of patient samples. Second, this study should aim to collect more samples from patients with renal disease, lymphoma, rheumatoid disease, and other diseases to determine the clinical utility of FLC in the diagnosis of various other diseases.
In conclusion, although sPEP/sIFE is the most commonly used assay for the diagnosis of MG and showed the best analytical and diagnostic performance among the methods tested, better diagnostic performance can be expected when used in combination with sFLC or uPEP/uFLC. The sFLC assay had a slightly lower diagnostic performance than sPEP/sIFE but showed a similar performance to uPEP/uIFE. It is expected that the sFLC assay can help in the diagnosis of MGs by overcoming the shortcomings and limitations of sPEP/sIFE when used along with sPEP/sIFE.
Acknowledgments
Dow Biomedica (Seoul, Korea), the Korean distributor of the Binding Site, Ltd. (Birmingham, UK), donated the reagents for free light chain (FLC) assays. This study was supported (in part) by a grant from the Yonsei University Future-Leading Research Initiative of 2021 (2021-52-0074).
REFERENCES
1. Sasson SC, McGill K, Wienholt L, et al. 2015; Comparison of the Freelite serum free light chain (SFLC) assay with serum and urine electrophoresis/immunofixation and the N Latex FLC assay. Pathology. 47:564–9. DOI: 10.1097/PAT.0000000000000316. PMID: 26352111.
2. Wijeratne N, Tate JR, Wienholt L, Mollee P. 2019; Report of the survey conducted by RCPAQAP on current practice for paraprotein and serum free light chain measurement and reporting: a need for harmonisation. Clin Biochem Rev. 40:31–42. PMID: 30828118. PMCID: PMC6370284.
3. Bradwell AR, Carr-Smith HD, Mead GP, et al. 2001; Highly sensitive, automated immunoassay for immunoglobulin free light chains in serum and urine. Clin Chem. 47:673–80. DOI: 10.1093/clinchem/47.4.673. PMID: 11274017.
4. Campos ML, Barbosa-de Carvalho NM, Martín-Reyes G. 2012; The value of serum free light chain assay in patients with monoclonal gammopathies and renal failure. Nefrologia. 32:15–9. DOI: 10.3265/Nefrologia.pre2011.Nov.11098. PMID: 22294000.
5. Bhole MV, Sadler R, Ramasamy K. 2014; Serum-free light-chain assay: clinical utility and limitations. Ann Clin Biochem. 51:528–42. DOI: 10.1177/0004563213518758. PMID: 24489083.
6. Nowrousian MR, Brandhorst D, Sammet C, et al. 2005; Serum free light chain analysis and urine immunofixation electrophoresis in patients with multiple myeloma. Clin Cancer Res. 11:8706–14. DOI: 10.1158/1078-0432.CCR-05-0486. PMID: 16361557.
7. Lobe M, Pasquale D. 2017; Freelite for measurement of urine-free light chains in monoclonal gammopathies. Am J Hematol Oncol. 12:9–13.
8. Snyder MR, Clark R, Bryant SC, Katzmann JA. 2008; Quantification of urinary light chains. Clin Chem. 54:1744–6. DOI: 10.1373/clinchem.2008.107599. PMID: 18824580.
9. Katzmann JA, Clark RJ, Abraham RS, et al. 2002; Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 48:1437–44. DOI: 10.1093/clinchem/48.9.1437. PMID: 12194920.
10. Erdem BK, Davran F, Yilmaz VT, Çetinkaya R, Akbas H. 2015; The association of serum-free light-chain levels with markers of renal function. Ren Fail. 37:1057–60. DOI: 10.3109/0886022X.2015.1052980. PMID: 26056734.
11. Katzmann JA, Kyle RA, Benson J, et al. 2009; Screening panels for detection of monoclonal gammopathies. Clin Chem. 55:1517–22. DOI: 10.1373/clinchem.2009.126664. PMID: 19520758. PMCID: PMC3773468.
12. Abraham RS, Clark RJ, Bryant SC, et al. 2002; Correlation of serum immunoglobulin free light chain quantification with urinary Bence Jones protein in light chain myeloma. Clin Chem. 48:655–7. DOI: 10.1093/clinchem/48.4.655. PMID: 11901068.
13. Singhal S, Stein R, Vickrey E, Mehta J. 2007; The serum-free light chain assay cannot replace 24-hour urine protein estimation in patients with plasma cell dyscrasias. Blood. 109:3611–2. DOI: 10.1182/blood-2006-11-060368. PMID: 17409349.
Fig. 1
Receiver operating characteristics curve analysis for the diagnostic agreement of ND/RR MG (AUC and 95% confidence intervals).
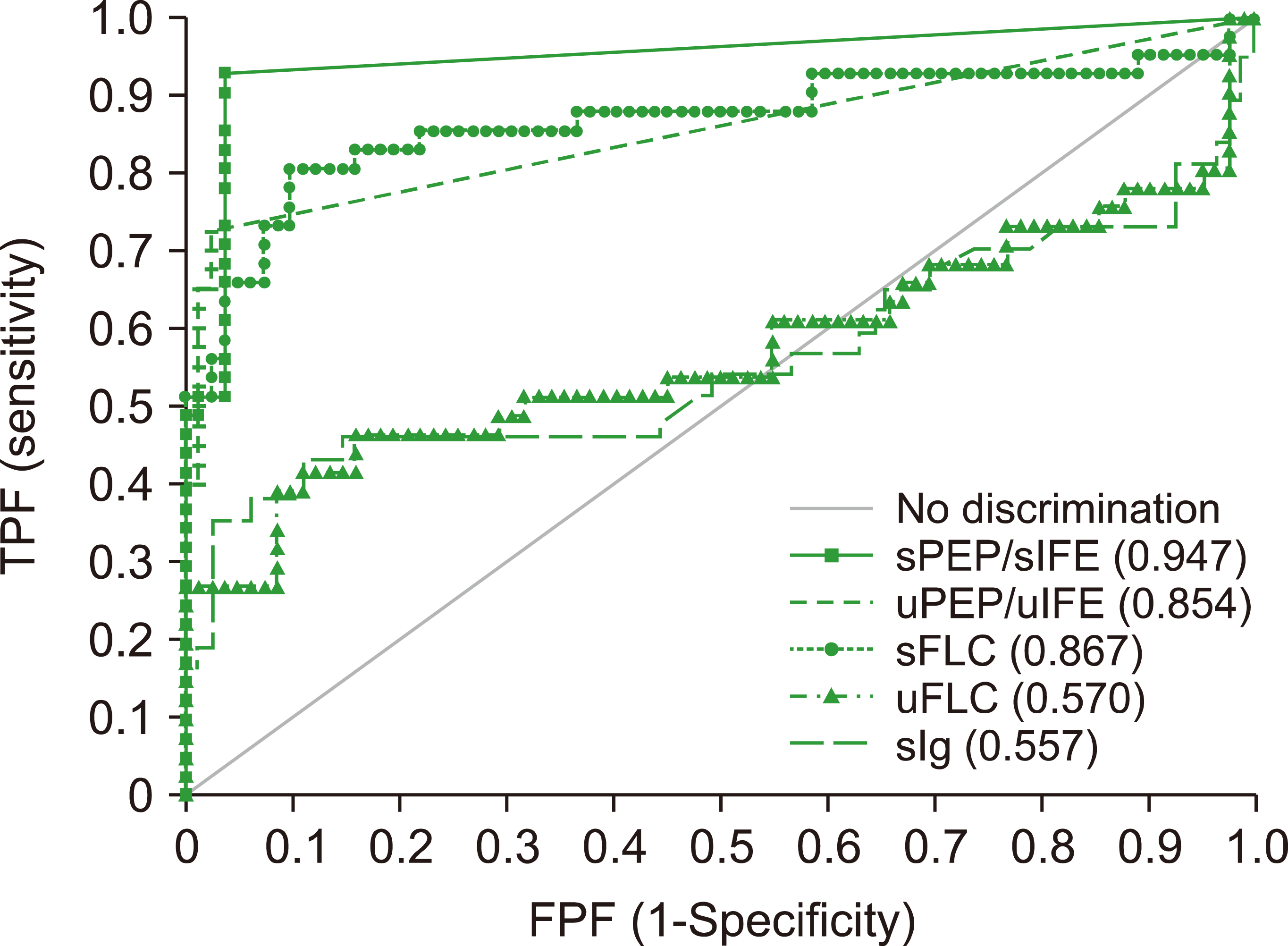
Table 1
Comparison of serum and urine free light chain values (unit: mg/L) determined by the Freelite assay according to the presence of ND/RR MG (median and interquartile ranges).
Table 2
Comparison of serum and urine free light chain values (unit: mg/L) in non-MG patients determined by the Freelite assay according to the eGFR ranges (median and 95% confidence intervals).
Table 3
Comparison of the analytical performance of serum and urine protein electrophoresis and free light chain assay for diagnosing ND/RR MG (mean and 95% confidence intervals).
Abbreviations: LR+, positive likelihood ratio; LR-, negative likelihood ratio; MG, monoclonal gammopathy; ND/RR, newly diagnosed/refractory or relapsed; NPV, negative predictive value; PPV, positive predictive value; sFLC, serum free light chain; sIg, serum immunoglobulin; sPEP/sIFE, serum protein electrophoresis and immunofixation electrophoresis; uFLC, urine free light chain; uPEP/uIFE, urine protein electrophoresis and immunofixation electrophoresis.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download