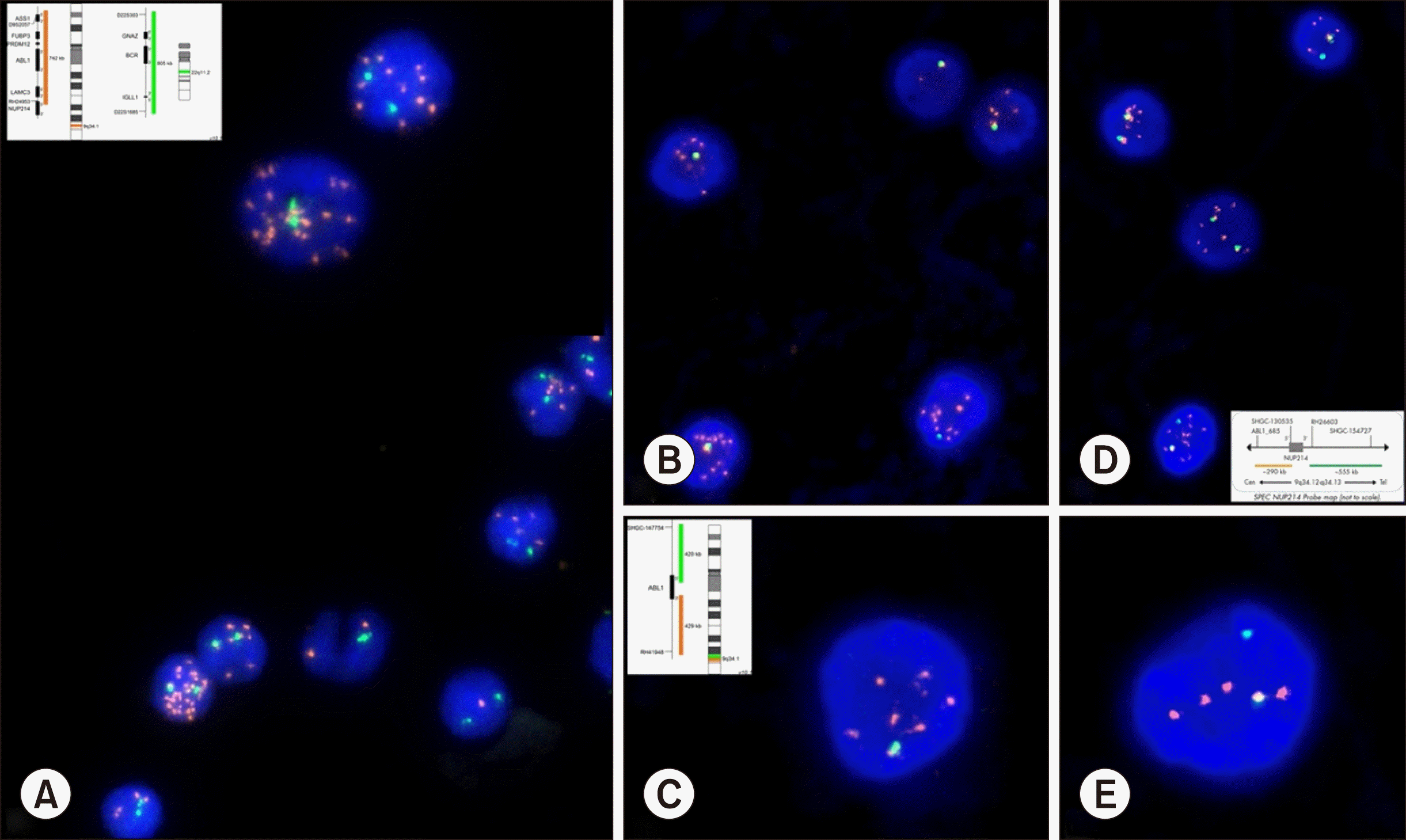
 | Fig. 1
(A) FISH using BCR-ABL1 dual-color dual-fusion probe showing two green signals (normal BCR gene) and multiple orange signals (3–50 copies), indicating amplification of the ABL1 gene; (B, C) FISH using ABL1 break-apart probe showing one fusion (normal ABL1 gene) and multiple orange signals, indicating amplification of the 3′ region of the ABL1 gene along with deletion of the 5′ region of the ABL1 gene; (D, E) FISH using NUP214 break-apart probe showing one fusion (normal NUP214 gene), one green (normal 3′ region of the other NUP214 allele), and multiple orange signals, indicating amplification of the 5′ region of the NUP214 gene.
Abbreviation: FISH, fluorescent in-situ hybridization.
|
TO THE EDITOR: NUP214-ABL1 translocation is probably the most common tyrosine kinase inhibitor (TKI)-targetable cytogenetic abnormality observed in T-cell acute lymphoblastic leukemia (T-ALL), accounting for 5–6% of the T-ALL cases [1]. However, there are only few case reports and small series in the literature, possibly indicating the underdiagnosis of this entity. Unlike in B-cell acute lymphoblastic leukemia, testing for gene fusions using reverse transcriptase polymerase chain reaction (RT-PCR) or fluorescent in-situ hybridization (FISH) is often avoided in T-ALL because of the absence of distinct associations with prognosis or targeted therapy, especially in resource-limited settings. However, TKI-targetable abnormalities have also been described in T-ALL, including NUP214-ABL1, BCR-ABL1, and mutations in STAT5b [2, 3]. Among these, FISH testing using BCR-ABL1 dual-color dual-translocation probes can help to identify not only BCR:ABL1 but also NUP214-ABL1 based on the characteristic patterns in FISH testing. This is an illustrative case highlighting an easy and cost-effective approach for routine detection of cytogenetic abnormalities.
A developmentally normal male child, appropriately immunized for his age, presented with continuous moderate-grade fever, progressive pallor, and mild hepatosplenomegaly since 10 days. Peripheral blood examination revealed that the hemoglobin level was 71 g/L; total leukocyte count, 45.3×109/L; and platelet count, 65×109/L. The peripheral blood film revealed 72% blasts, 9% neutrophils, 16% lymphocytes, and 3% monocytes. The blasts were negative for myeloperoxidase. Flow cytometry confirmed T-ALL [positive for cluster of differentiation (CD) 7, CD4, CD5, CD2, cytoCD3, and terminal deoxynucleotidyl transferase (TdT); negative for surface CD3, CD1a, CD8, T-cell receptor (TCR) ab, TCRgd, B cell, and myeloid antigens]. FISH testing using BCR-ABL1 dual-color dual-fusion probe (Metasystems, Germany) showed amplification of ABL1 (3–50 copies) using the protocol as previously described [4]. Further testing using ABL1 (Metasystems, Germany) and NUP214 (Zytolight, Germany) dual-color break-apart probes showed a characteristic signal pattern confirming NUP214-ABL1 translocation (Fig. 1). Details of immunophenotyping and FISH cytogenetics are summarized in Table 1.
Hematological and laboratory parameters at diagnosis and after induction therapy.
Bone marrow examination was not performed, as a confirmatory diagnosis could be made from the peripheral blood investigation, and the child was not willing to undergo bone marrow examination. Conventional cytogenetics of the peripheral blood did not show metaphase. Augmented Berlin-Frankfurt-Munich protocol plus imatinib was administered. Post-induction bone marrow was hypocellular with 2% blasts, and no measurable residual disease was detected using 10-color flow-cytometric immuno-phenotyping. The delayed intensification phase 2 was completed uneventfully, and the child is now in the maintenance phase.
Both ABL1 and NUP214 genes are located at 9q34.1, with the latter on the telomeric side. The fusion of these genes results from extrachromosomal episome formation and amplification of both genes. The episome containing the fused gene exists autonomously and freely replicates in the cytoplasm or integrates with the chromosome and replicates with it. This episomal amplification, varying between 5–50 copies/cell, can be visualized using FISH, multiplex ligation-dependent probe amplification, or chromosomal microarray; however, it is undetectable with conventional cytogenetics. Amplification of ABL1 does not appear to be the only mechanism involved in the pathogenesis of T-ALL; there have been reports of associated alterations of other genes, such as CDKN2A, TLX1, TLX3, and NOTCH1. These observations indicate a multigene contribution to the pathogenesis of T-ALL with NUP214-ABL1 fusion. NUP214-ABL1 fusion is found predominantly in men, and these patients usually present with high-risk factors, including elevated leukocyte count, mediastinal mass, or extramedullary involvement, often with early relapse and dismal outcomes [5]. While an occasional patient has survived for more than 194 months, the median overall survival reported in previous series is only 18 months. These patients are reported to benefit from TKI, especially dasatinib; hence, it is imperative to diagnose this entity in the clinics [6]. However, the long-term benefit of adding TKI in the treatment remains unclear owing to the lack of randomized controlled trials. A summary of the cases reported until now is presented in Table 2.
The limited number of reports and absence of definite treatment guidelines may indicate underdiagnosis of this entity. Amplification of ABL1 (9q34) is an indirect indicator of NUP214-ABL1 fusion, which can easily be detected by routine testing of BCR-ABL1 using FISH in T-ALL cases. NUP214-ABL1 fusion can be further confirmed using NUP214 break-apart FISH testing or RT-PCR. In addition to BCR-ABL1 translocation, this dual-color probe FISH helps to detect other ABL1-related and possibly TKI-responsive cytogenetic abnormalities associated with T-ALL, such as EML1-ABL1 and ETV6-ABL1 fusions or 9q34 duplication associated with therapeutic resistance [7]. Although BCR-ABL1-positive T-ALL is rare, the frequency of its detection has increased, with approximately 30 cases reported in the literature and pediatric cases accounting for more than 40% of the total cases. In most reported cases, the prognosis was poor with a median survival of only 7 months (range, 0.1–60 mo), and nearly 50% of the patients died by the time of the last follow-up [8-15].
To summarize, this illustrative report highlights the utility of incorporating routine FISH testing using a BCR/ABL1 dual-color probe in the workup for T-ALL in resource-constrained settings, especially in the absence of advanced testing, such as ribonucleic acid -sequencing.
Acknowledgments
This study was supported by a grant from the National Cancer Grid, Government of India.
Go to : 
Notes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Go to : 
REFERENCES
1. Graux C, Cools J, Melotte C, et al. 2004; Fusion of NUP214 to ABL1 on amplified episomes in T-cell acute lymphoblastic leukemia. Nat Genet. 36:1084–9. DOI: 10.1038/ng1425. PMID: 15361874.
2. Ragg S, Zehentner BK, Loken MR, Croop JM. 2019; Evidence for BCR/ABL1-positive T-cell acute lymphoblastic leukemia arising in an early lymphoid progenitor cell. Pediatr Blood Cancer. 66:e27829. DOI: 10.1002/pbc.27829. PMID: 31136068.
3. Govaerts I, Jacobs K, Vandepoel R, Cools J. 2019; JAK/STAT pathway mutations in T-ALL, including the STAT5B N642H mutation, are sensitive to JAK1/JAK3 inhibitors. Hemasphere. 3:e313. DOI: 10.1097/HS9.0000000000000313. PMID: 31976485. PMCID: PMC6924561. PMID: 162e9a8a08404fc39958e4847a829457.
4. Sharma P, Rana S, Sreedharanunni S, et al. 2021; An evaluation of a fluorescence in situ hybridization strategy using air-dried blood and bone-marrow smears in the risk stratification of pediatric B-lineage acute lymphoblastic leukemia in resource-limited settings. J Pediatr Hematol Oncol. 43:e481–5. DOI: 10.1097/MPH.0000000000001892. PMID: 32769569.
5. Graux C, Stevens-Kroef M, Lafage M, et al. 2009; Heterogeneous patterns of amplification of the NUP214-ABL1 fusion gene in T-cell acute lymphoblastic leukemia. Leukemia. 23:125–33. DOI: 10.1038/leu.2008.278. PMID: 18923437.
6. Deenik W, Beverloo HB, van der Poel-van de Luytgaarde SC, et al. 2009; Rapid complete cytogenetic remission after upfront dasatinib monotherapy in a patient with a NUP214-ABL1-positive T-cell acute lymphoblastic leukemia. Leukemia. 23:627–9. DOI: 10.1038/leu.2008.318. PMID: 18987655.
7. Li X, Ping N, Wang Y, et al. 2021; Case report: a case with Philadelphia chromosome positive T-cell lymphoblastic lymphoma and a review of literature. Front Oncol. 10:584149. DOI: 10.3389/fonc.2020.584149. PMID: 33552960. PMCID: PMC7857119. PMID: 1ca4c8184a7a44399fad867d173ab6c0.
8. Jain P, Kantarjian H, Jabbour E, et al. 2017; Clinical characteristics of Philadelphia positive T-cell lymphoid leukemias-(De novo and blast phase CML). Am J Hematol. 92:E3–4. DOI: 10.1002/ajh.24579. PMID: 27727470. PMCID: PMC5551407.
9. Ballerini P, Busson M, Fasola S, et al. 2005; NUP214-ABL1 amplification in t(5;14)/HOX11L2-positive ALL present with several forms and may have a prognostic significance. Leukemia. 19:468–70. DOI: 10.1038/sj.leu.2403654. PMID: 15674415.
10. Barber KE, Martineau M, Harewood L, et al. 2004; Amplification of the ABL gene in T-cell acute lymphoblastic leukemia. Leukemia. 18:1153–6. DOI: 10.1038/sj.leu.2403357. PMID: 15057249.
11. Kim HJ, Woo HY, Koo HH, Tak EY, Kim SH. 2004; ABL oncogene amplification with p16(INK4a) gene deletion in precursor T-cell acute lymphoblastic leukemia/lymphoma: report of the first case. Am J Hematol. 76:360–3. DOI: 10.1002/ajh.20117. PMID: 15282669.
12. Chen Y, Zhang L, Huang J, et al. 2017; Dasatinib and chemotherapy in a patient with early T-cell precursor acute lymphoblastic leukemia and NUP214-ABL1 fusion: a case report. Exp Ther Med. 14:3979–84. DOI: 10.3892/etm.2017.5046. PMID: 29067094. PMCID: PMC5647690.
13. Peterson JF, Pitel BA, Smoley SA, et al. 2019; Detection of a cryptic NUP214/ABL1 gene fusion by mate-pair sequencing (MPseq) in a newly diagnosed case of pediatric T-lymphoblastic leukemia. Cold Spring Harb Mol Case Stud. 5:a003533. DOI: 10.1101/mcs.a003533. PMID: 30936193. PMCID: PMC6549564.
14. De Keersmaecker K, Lahortiga I, Graux C, et al. 2006; Transition from EML1-ABL1 to NUP214-ABL1 positivity in a patient with acute T-lymphoblastic leukemia. Leukemia. 20:2202–4. DOI: 10.1038/sj.leu.2404425. PMID: 17024111.
15. Bernasconi P, Calatroni S, Giardini I, et al. 2005; ABL1 amplification in T-cell acute lymphoblastic leukemia. Cancer Genet Cytogenet. 162:146–50. DOI: 10.1016/j.cancergencyto.2005.04.002. PMID: 16213363.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download