Abstract
Background
Factor VIII (FVIII) inhibitor diagnosis and surveillance in Indonesia are challenging owing to geographic conditions and the lack of laboratory facilities nationwide for inhibitor assays. This study aimed to determine the prevalence of FVIII inhibitors in children diagnosed with hemophilia A (HA) in Indonesia.
Methods
A cross-sectional study was conducted in 12 hospitals in eight provinces of Indonesia between 2020 and 2021. Factor VIII inhibitor screening was performed in a central hemostasis laboratory for all children with HA (≤18 yr) who had received a minimum of 10 exposure days to clotting factor concentrates. The FVIII inhibitor titer was determined using the Bethesda assay.
Results
Children (388) were enrolled in this study, including 219 (56.4%), 131 (33.8%), and 38 (9.4%) with severe, moderate, and mild HA, respectively. The prevalence of children who developed FVIII inhibitors was 37 out of 388 (9.6%). Factor VIII inhibitors were found in 25/219 (11.4%) severe, 11/131 (8.3%) moderate, and 1/38 (2.6%) children with mild HA. Thirteen children had low-titer inhibitors and 24 had high-titer inhibitors, with a median of 9.44 (1.48‒412.0) Bethesda Units. Among 13 children with low-titer inhibitors, eight underwent a confirmation test, of which five tested negative and were classified as transient. A significant difference in annual joint bleeding rate was found between patients with low and high inhibitor titers and those without inhibitors (P<0.001).
Hemophilia A (HA) is an X-linked recessive bleeding disorder characterized by factor VIII (FVIII) deficiency [1]. The standard treatment for HA is FVIII replacement therapy, which may be administered prophylactically or on demand. Repeated administration of exogenous FVIII concentrates can result in antibody formation, which is called an inhibitor [1, 2]. Inhibitors are most frequently encountered in patients with severe HA (20–30%) [3, 4], with the highest incidence occurring during the first 20–30 days of exposure [5, 6]. A post-hoc analysis of the Study on Inhibitors in Plasma-Product Exposed Toddlers (SIPPET) also revealed that the highest rate of inhibitor development occurred during the first 10 days of exposure [7, 8]. The development of an FVIII inhibitor complicates bleeding management in patients with HA and increases morbidity, mortality, and treatment cost [1, 4].
To date, available data regarding the prevalence of FVIII inhibitors in HA patients in lower-middle-income countries with healthcare constraints, such as Indonesia, are limited. Although the diagnosis and management of patients with hemophilia in Indonesia have improved in recent years, there are still several unmet needs. Factor VIII inhibitor assays are not widely available in hemophilia treatment centers across all the provinces of Indonesia. Limited access to healthcare facilities owing to geographic and socio-economic conditions and the high expense of bypassing agents remain challenges for treating patients with hemophilia with FVIII inhibitors. The data on FVIII inhibitor prevalence are valuable to healthcare providers and policymakers for improving the treatment of patients with hemophilia. This study aimed to determine the prevalence of FVIII inhibitors among children diagnosed with HA in Indonesia.
This was a multicenter, descriptive study involving eight provinces in Indonesia (Jakarta, Banten, West Java, East Java, Central Java, Yogyakarta, North Sumatra, and South Sumatra) conducted from January 2020 to October 2021. The inclusion criteria were children diagnosed with HA, aged ≤18 years, who had received a minimum of 10 days of exposure to clotting factor concentrate (CFC) before FVIII inhibitor testing. Patients who refused to undergo FVIII inhibitor testing were excluded.
Baseline data, including patient demographics, severity of hemophilia, family history of FVIII inhibitors, annual bleeding rate (ABR), annual joint bleeding rate (AJBR), history of major bleeding, and surgery before FVIII inhibitor development, were obtained from medical records. Ethical approval was obtained from The Ethics Committee of the Faculty of Medicine, Universitas Indonesia, Dr. Cipto Mangunkusumo Hospital (No. 1199/UN2.F1/Ethics/PPM.00. 02/2021).
The FVIII inhibitor assay was performed in a central hemostasis laboratory (Department of Clinical Pathology, Dr. Cipto Mangunkusumo Hospital, Jakarta). To deliver samples from hospitals in other provinces, we collaborated with a private laboratory (Prodia) with nationwide coverage and branches in every province, using a standardized referral sample delivery system. During outpatient clinic visits, patients were asked to visit the selected Prodia laboratory in their provinces. Blood samples were collected from the peripheral vein and placed in a citrate-filled plastic tube with a blood to anticoagulant ratio of 1:9. Blood samples were centrifuged at 1,700×g for 15 min at room temperature (15–25°C) to obtain a plasma citrate specimen, which was then frozen at -20°C. The frozen 1 mL plasma citrate specimens were sent to the Prodia laboratory in Jakarta using dry ice packs to maintain the temperature at -20°C. The frozen samples were then referred to the Laboratory Department of Clinical Pathology, Dr. Cipto Mangunkusumo Hospital, Jakarta. The time from sample collection to the Bethesda assay was less than 1 week. The FVIII inhibitor titer was measured using the Bethesda assay. A factor VIII inhibitor was considered negative if the titer was <0.6 Bethesda Units (BU). A low-titer inhibitor had a Bethesda titer of between ≥0.6 and <5 BU, and ≥5 BU was considered a high-titer inhibitor.
Data were analyzed using the Statistical Package for Social Sciences (SPSS, IBM Corporation, Armonk, NY, USA) version 26.0. Categorical data are presented as frequencies and percentages in tables or graphs. Numerical data with normal distribution are provided as the mean±standard deviation (SD), and data with skewed distribution are provided as the median and lower-upper interquartile range (Q1; Q3). We also conducted a chi-square test to determine whether there were significant differences in patients’ characteristics according to the inhibitor titer. Pearson’s exact test was used if the data did not fulfill the chi-squared criteria. Post-hoc testing was conducted using pairwise Z-test statistical analysis between patients with and without inhibitors.
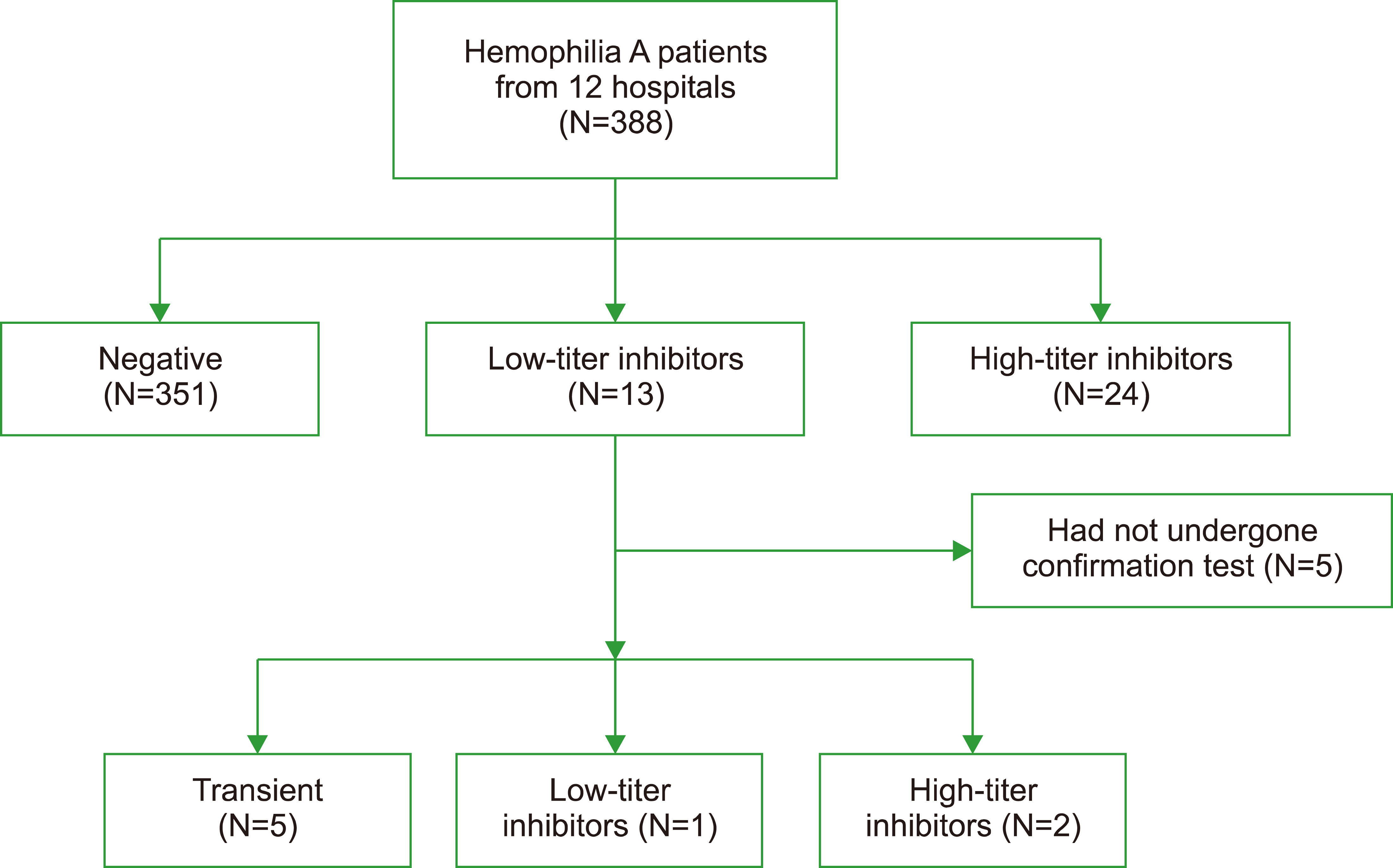
Between 2020 and 2021, the total number of patients with HA aged ≤18 years registered in the Indonesian Hemophilia Society (IHS) national registry was 1,336. A total of 388 patients with HA from 12 hospitals in eight provinces participated in this study. Almost all patients received plasma-derived FVIII concentrates because of the availability of the products. During the study period, recombinant factors were only available from donations by the World Federation of Hemophilia (WFH) (Table 1). Factor VIII inhibitors were detected in 9.6% (37) of children with HA in the 12 hospitals in Indonesia. The proportion of each inhibitor titer is shown in Fig. 1. The median FVIII inhibitor titer was 9.44 (1.48–412.0) BU. The median age of patients treated with FVIII inhibitors was 8 (5.5; 8.5) years. Factor VIII inhibitors were the most common in patients with severe HA (67.6%), with the majority having high-titer inhibitors.
Among all patients with FVIII inhibitors, only 12 had a history of switching products to recombinant FVIII owing to a clinical event (Table 2). During their lifetime, 16 of the 37 patients with FVIII inhibitors underwent the following surgical procedures: circumcision in 12 patients (between 2015 and 2019), tooth extraction in one patient in 2015, appendicectomy in one patient in 2018, and more than one surgical procedure in two patients. The median period between the surgical procedures and FVIII inhibitor development in these patients was 2.5 (1–12) years. We did not find significant differences in any baseline characteristics between patients with low and high inhibitor titers (Table 2).
Five patients who developed FVIII inhibitors also had a history of major bleeding: four patients had an intracranial hemorrhage (2017–2019) and one patient had an iliopsoas hemorrhage in 2020 when FVIII inhibitors were diagnosed. The median period between major bleeding events and FVIII inhibitor testing was 2 (0–15) years. Most patients with FVIII inhibitors also had target joints, with the knee and ankle being the most affected. The characteristics of the patients treated with FVIII inhibitors are shown in Table 2. The ABR and AJBR of patients without inhibitors, low-titer inhibitors, and high-titer inhibitors are presented in Table 3. Our results showed a significant difference in AJBR between patients with low and high inhibitor titers and those without inhibitors (P<0.001).
Of the 13 patients with low-titer inhibitors, eight underwent a confirmation test approximately 6 months after the baseline test, whereas the remaining five were not further tested (Fig. 1). In the confirmation test, five patients tested negative, two patients had high-titer inhibitors (6.4 BU and 5.2 BU), and one had a low-titer inhibitor (2.68 BU).
This study included 29.8% children with HA aged ≤18 years who were registered in the IHS National Registry. Based on the IHS data, approximately 60% of these patients lived in small cities and rural areas in the eight provinces that participated in this study. To participate, patients were asked to visit the capital city of each province. However, the COVID-19 pandemic and difficult access to transportation resulted in a low number of patients participating in our study. The prevalence of FVIII inhibitors among patients with severe HA in our study (25/219, 12%)
The management of patients with inhibitors includes the treatment of bleeding episodes, eradication of inhibitors through induction of immune tolerance, and prophylaxis. Immune tolerance induction and prophylaxis with non-factor replacement therapy (emicizumab) are not feasible in Indonesia because of prohibitive costs. All children diagnosed with inhibitors in Indonesia were treated with bypassing agents, mostly donated by the WFH Humanitarian Aid Program.
The WFH recommends routine inhibitor screening during the period with the highest risk of inhibitor development; that is, at least every 6–12 months after CFC therapy is initiated and annually following treatment. Screening is recommended for all patients with hemophilia, regardless of age or disease severity [1]. However, because of the limited facilities and financial constraints in Indonesia, FVIII inhibitor testing is only performed before surgery, after intensive exposure to CFC, or in patients who fail to respond to adequate CFC treatment. Moreover, the FVIII inhibitor assay is unavailable for routine daily services in provinces outside Jakarta.
From a technical perspective, shipping samples to a central laboratory is also challenging. Samples that cannot be transported as whole blood to the referred laboratory should be processed by centrifugation, ideally within 4 h of collection [13, 14]. If samples are stored for more than 4 h after collection, they should be maintained in an appropriate freezer (-20°C) and tests performed within 2 weeks or less [13, 14]. Using this method, patients from outside Jakarta could be tested for FVIII inhibitors. We collaborated with a private laboratory to collect and deliver samples to the central laboratory. However, in daily practice, the national insurance plan does not cover transportation costs, which is a burden on patients.
Over the last decade, several variable risk factors have been analyzed to understand how they might contribute to the development of FVIII inhibitors, such as FVIII products, age at first exposure, intensity of treatment, “danger signals” caused by surgery, major bleeding events, vaccination, or infection [5, 15]. In the current study, 32.4% of patients with FVIII inhibitors had a history of switching products to recombinant FVIII, 13.5% had a major bleeding event, and 43.2% had a history of surgery, 1 year before FVIII inhibitor testing was performed (Table 2) During surgical procedures or major bleeding events, patients received intense treatment exposure, defined as five or more consecutive days of treatment, resulting in an increased risk of inhibitor development [8]. However, given the retrospective nature of this study, it was difficult to collect comprehensive data regarding the treatment type and intensity, time between the suspected events, and exact time for the initial development of the FVIII inhibitor.
Muscle and joint bleeding are the main clinical presentations of severe hemophilia, and their burden is greater in patients with inhibitors than in those without [2, 10]. Some factors, including patient compliance, referral problems to hemophilia centers, and large-scale social restrictions during the COVID-19 pandemic in some provinces, affected the data collection in this study. As shown in Table 3, the inhibitor titer was significantly associated with the AJBR (P<0.001). According to post-hoc analysis, the number of patients with AJBR 13–24x/year was significantly higher in patients with a low-titer inhibitor than in patients without inhibitors. In patients with AJBR 25–48x/year, we found a higher proportion of patients without inhibitors than in patients with a high titer of inhibitors [112 out of 351 (31.9%) versus 4 out of 24 (16.7%), respectively]. This result may be owing to differences in age between the two groups [median of 10 (range, 5–18) years in patients without inhibitor versus 6 (range, 5–12) years in patients with a high titer inhibitor], which led to more target joints in 62/112 (55.4%) among group of patients without inhibitors. Of those who developed the target joint, 47/62 (75.8%) had more than one target joint and 15/62 (24.2%) had one target joint.
Low-titer inhibitors tend to be transient and often resolve within 3–6 months, whereas high-titer inhibitors are usually persistent [1, 16]. In our study, a confirmation test was performed approximately 6 months after the baseline test; among the 13 patients with low-titer inhibitors, eight patients underwent a confirmation test, of which five patients had negative results and were classified as transient. However, five patients did not undergo a confirmation test because of patient compliance and limited access to the study center during the COVID-19 pandemic.
Our study establishes a basis for further research to manage and understand the challenges in diagnosing FVIII inhibitors, particularly in developing countries. The limitation of this study was the lack of further analysis of contributing variables, such as FVIII genetic mutations and other thrombotic disorders, and their relationship to the development of FVIII inhibitors. However, this could be an objective of future research to improve hemophilia care in Indonesia.
ACKNOWLEDGEMENTS
We thank the Indonesian Hemophilia Society for their support in reaching out to hemophilia patients living in the cities/provinces participating in this study and the Prodia Laboratory for their assistance in collecting and delivering blood samples. The FVIII inhibitor surveillance project was funded by Roche Indonesia. No honoraria were received by the authors for the manuscript writing
REFERENCES
1. Srivastava A, Santagostino E, Dougall A, et al. 2020; WFH Guidelines for the Management of Hemophilia, 3rd edition. Haemophilia. 26:1–158. DOI: 10.1111/hae.14046. PMID: 32744769.
2. Carcao M, Goudemand J. 2018. Inhibitors in hemophilia: a primer. 5th ed. World Federation of Hemophilia;Montréal, Canada: at http://www1.wfh.org/publication/files/pdf-1122.pdf. Accessed November 29, 2021.
3. Villarreal-Martínez L, García-Chávez J, Sánchez-Jara B, et al. 2020; Prevalence of inhibitors and clinical characteristics in patients with haemophilia in a middle‐income Latin American country. Haemophilia. 26:290–7. DOI: 10.1111/hae.13951. PMID: 32141696.
4. Ljung R, Auerswald G, Benson G, et al. 2019; Inhibitors in haemophilia A and B: management of bleeds, inhibitor eradication and strategies for difficult‐to‐treat patients. Eur J Haematol. 102:111–22. DOI: 10.1111/ejh.13193. PMID: 30411401. PMCID: PMC6936224.
5. Garagiola I, Palla R, Peyvandi F. 2018; Risk factors for inhibitor development in severe hemophilia A. Thromb Res. 168:20–7. DOI: 10.1016/j.thromres.2018.05.027. PMID: 29879570. PMCID: PMC6055565.
6. Mancuso ME, Fischer K, Santagostino E, et al. 2017; Risk factors for the progression from low to high titres in 260 children with severe haemophilia A and newly developed inhibitors. Thromb Haemost. 117:2274–82. DOI: 10.1160/TH17-01-0059. PMID: 29212115.
7. Peyvandi F, Cannavò A, Garagiola I, et al. 2018; Timing and severity of inhibitor development in recombinant versus plasma-derived factor VIII concentrates: a SIPPET analysis. J Thromb Haemost. 16:39–43. DOI: 10.1111/jth.13888. PMID: 29080391.
8. Peyvandi F, Garagiola I. 2018; Product type and other environmental risk factors for inhibitor development in severe hemophilia A. Res Pract Thromb Haemost. 2:220–7. DOI: 10.1002/rth2.12094. PMID: 30046724. PMCID: PMC6055565.
9. Collins PW, Palmer BP, Chalmers EA, et al. 2014; Factor VIII brand and the incidence of factor VIII inhibitors in previously untreated UK children with severe hemophilia A, 2000-2011. Blood. 124:3389–97. DOI: 10.1182/blood-2014-07-580498. PMID: 25339360. PMCID: PMC4246037.
10. Peyvandi F, Kavakli K, El‐Beshlawy A, Rangarajan S. 2022; Management of haemophilia A with inhibitors: a regional cross‐talk. Haemophilia. 28:950–61. DOI: 10.1111/hae.14638. PMID: 35868021.
11. Volkers P, Hanschmann KM, Calvez T, et al. 2019; Recombinant factor VIII products and inhibitor development in previously untreated patients with severe haemophilia A: combined analysis of three studies. Haemophilia. 25:398–407. DOI: 10.1111/hae.13747. PMID: 31066174.
12. Gouw SC, van der Bom JG, Ljung R, et al. 2013; Factor VIII products and inhibitor development in severe hemophilia A. N Engl J Med. 368:231–9. DOI: 10.1056/NEJMoa1208024. PMID: 23323899.
13. Adcock Funk DM, Lippi G, Favaloro EJ. 2012; Quality standards for sample processing, transportation, and storage in hemostasis testing. Semin Thromb Hemost. 38:576–85. DOI: 10.1055/s-0032-1319768. PMID: 22706973.
14. Kitchen S, Adcock DM, Dauer R, et al. 2021; International Council for Standardization in Haematology (ICSH) recommendations for processing of blood samples for coagulation testing. Int J Lab Hematol. 43:1272–83. DOI: 10.1111/ijlh.13702. PMID: 34581008.
15. Schep SJ, Boes M, Schutgens REG, van Vulpen LFD. 2019; An update on the 'danger theory' in inhibitor development in hemophilia A. Expert Rev Hematol. 12:335–44. DOI: 10.1080/17474086.2019.1604213. PMID: 30951401.
16. Meeks SL, Batsuli G. 2016; Hemophilia and inhibitors: current treatment options and potential new therapeutic approaches. Hematology Am Soc Hematol Educ Program. 2016:657–62. DOI: 10.1182/asheducation-2016.1.657. PMID: 27913543. PMCID: PMC6142469.
Table 1
Patient demographics.
Table 2
Characteristics of patients with FVIII Inhibitors.
a)1 year before FVIII inhibitor testing. b)Plasma-derived products used are Koate DVI, Haemoctin, Octanate. c)History of switching products from plasma-derived to recombinant due to a clinical event. All recombinant FVIII concentrates were donated by the World Federation of Hemophilia’s Humanitarian Aid Program. d)P-value is calculated using Pearson’s exact test.
Table 3
Annual Bleeding Rate (ABR) and Annual Joint Bleeding Rate (AJBR) by inhibitor baseline.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download