Abstract
Objective
Rathke’s cleft cysts (RCCs) are nonneoplastic cysts. Most of them are asymptomatic and stable; when symptomatic, RCCs are surgically fenestrated and drained. However, the outcomes remain unclear. The authors evaluated the outcomes of RCC decompression.
Methods
Between 2004 and 2019, 32 RCCs were decompressed in a single tertiary institution. The clinical characteristics, intraoperative findings, postoperative complications, and endocrinological and surgical outcomes were retrospectively reviewed. Patients who underwent sequential imaging at least twice and at least 12 months after surgery were included in the analysis.
Results
Patients’ mean age was 40.8±14.9 years, and 62.5% were women. The mean follow-up duration was 62.3±48.6 months. In 21 patients (65.6%), no residual cysts were identified on postoperative magnetic resonance imaging. Of the 18 patients with preoperative visual field defects, 17 (94.4%) experienced postoperative visual improvement. Postoperative complications included endocrinological deterioration in 11 patients (34.4%), permanent diabetes insipidus in 11 (34.4%), infection in four (12.5%), intrasellar hemorrhage in three (9.4%), and cerebrospinal fluid leak in two (6.3%). Follow-up images revealed cyst recurrence in nine patients (28.1%), an average of 20.4 months after surgery; in three patients, the cysts were symptomatic, and resection was repeated. Multivariable analysis revealed that postoperative endocrinological deterioration was the only independent factor associated with cyst recurrence (p=0.028; hazard ratio, 6.800).
Conclusion
Our findings showed that although only cyst fenestration for decompression was performed to preserve pituitary function, more pituitary dysfunction occurred than expected. Besides, the postoperative hormonal deterioration itself acted as a risk factor for cyst recurrence. In conclusion, surgery for RCC should be more careful.
Go to : 
Rathke’s cleft cysts (RCCs) are nonneoplastic lesions that arise from embryological remnants of the pars intermedia within Rathke’s pouch [18]. They are common intrasellar and suprasellar lesions and are found incidentally in 4–33% of routine autopsies [18,23,25]. The majority of RCCs are asymptomatic and stable. Symptomatic RCCs may cause headache, nausea, visual impairment, and endocrinopathy [11]. Symptomatic RCCs are usually treated surgically, and most symptoms resolve postoperatively [15].
As a result of improvements in neuroimaging techniques, more RCCs have been diagnosed, and their natural course is better understood [19,21]. Furthermore, extensive experience with endoscopic transsphenoidal surgery has elucidated the surgical anatomy of the sellar region and is believed to have improved outcomes somewhat [10,14]. However, the relative merits of complete cyst wall resection remain controversial; few surgical outcomes have been reported. The aim of this study was to evaluate the outcomes of patients undergoing surgical resection of RCCs and thereby determine optimal strategies for treating RCCs.
Go to : 
The study complied with the Declaration of Helsinki. Institutional Review Board of Seoul National University Bundang Hospital approved the current study (No. B-2108-701-106) and waived the requirement for written informed consent.
We retrospectively assessed patients who underwent surgical evacuation of RCCs in our hospital, between 2004 and 2019. During this period, RCCs were diagnosed in 580 patients in that department. The medical records were retrieved from a database of patients who either were under “wait-andsee” management or had undergone surgical treatment. Data collected included electronic medical records, findings of endocrinological and ophthalmological examinations, imaging studies, operation notes, and pathology reports.
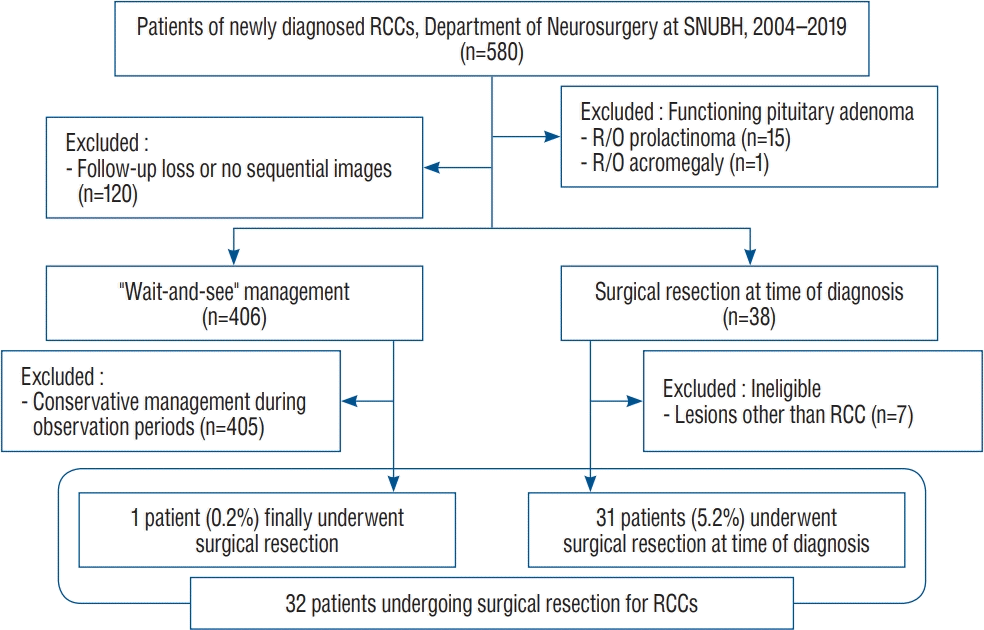
The subjects of this study were patients who underwent surgery for RCCs with symptoms and had adequate follow-up durations to evaluate the surgical results. Our inclusion criteria of this study were as follows : 1) surgically managed RCCs with pathological confirmation by a neuropathologist; 2) no clinical or biochemical evidence of endocrinologically functioning tumors; 3) endocrinological and ophthalmological examination before and after surgery; and 4) after surgery, at least two sequential magnetic resonance imaging (MRI) scanned at intervals of at least 12 months during the follow-up period. We excluded patients with abnormal endocrinological results suggestive of functioning pituitary adenomas, such as prolactinoma or acromegaly, and patients in whom diseases other than RCCs were diagnosed after surgery. For hyperprolactinemia, other causes rather than stalk-effect by RCCs were ruled out through further diagnostic test such as dilution test. All cases with endocrinological abnormality were discussed with endocrinologist. Of the 580 patients with diagnosed RCCs, 120 were excluded because imaging follow-up was not performed or because medical records were incomplete, and 16 were excluded because hormone study results were suggestive of functioning pituitary adenomas. Of the remaining 444 patients, 38 patients underwent surgery but seven were excluded because diseases other than RCCs were confirmed after surgery. Of 406 patients observed on a wait-and-see” management with a mean follow-up period of 29.4 months, only one (0.2%) eventually underwent surgical resection (Fig. 1). We studied surgical outcomes in this patient and the 31 other patients who underwent surgery.
Standard follow-up monitoring of the sellar lesions at our hospital comprised MRI, endocrinological examination, and ophthalmological examination when symptoms were suspected to be related, as well as routine neurological examination.
Within a week after surgery, patients underwent follow-up MRI of the sellar region. If no evidence of residual cysts was found, MRI was repeated annually for 2 years and approximately every 2 years thereafter. If a residual cyst was noted, MRI was repeated every 6–9 months for up to 2 years and then annually for the next 5 years if the cyst showed no evidence of enlargement. If any symptoms of visual deterioration or other neurological aggravation developed during the follow-up visits, the patients underwent MRI and ophthalmological examination immediately.
Endocrinological evaluation was conducted at the time of diagnosis and during the postoperative follow-up visits, in which MRI was performed. For hormone studies, radioimmunoassay and immunoradiometric assays between 8 and 10 a.m. were performed to measure basal levels of growth hormone (GH), insulin-like growth factor-I, adrenocorticotropic hormone (ACTH), serum cortisol, free T4, thyroid-stimulating hormone (TSH), prolactin, luteinizing hormone, folliclestimulating hormone, estradiol, and total testosterone. A rapid ACTH stimulation test was performed in patients with suspected adrenal insufficiency, as previously described [6]. Endocrinological deterioration was defined as a loss of ≥1 of the hormonal axis during the follow-up period, and improvement in endocrine function was defined as a gain of ≥1 of the hormonal axis during the follow-up. Loss or gain of hormonal axis was defined biochemically, not by whether hormone replacement was initiated. All patients with endocrine dysfunction, whether preoperatively or postoperatively, were referred to an endocrinologist for a thorough assessment and medical management.
At the time of diagnosis, ophthalmological examination, including a test of visual acuity and a visual field test, was performed. To evaluate the visual field, the Goldmann visual field test or the Humphrey visual field analyzer (Humphrey Field Analyzer II 750; Carl Zeiss Meditec, Dublin, OH, USA) was used. Patients with visual impairment underwent careful ophthalmological assessment 1 month after surgery. Visual aggravation was defined as any further impairment of visual acuity or visual field.
Surgical strategies for RCCs at our hospital are conservative. The surgical goal is decompression through cyst fenestration, not the evacuation of the cyst wall. Biopsy of the cyst wall was conducted via surgical corridor. The extent of resection was measured on MRI within 1 week after surgery. The procedure was defined as no residual cyst if there was no cyst on postoperative MRI. If any cysts were left, the procedure was classified as residual cyst. When cyst recurrence was suspected during the follow-up period, reoperation was considered only if symptoms were thought to be caused by RCC. Patients without symptoms related to recurrent RCCs were kept on observation without any intervention, even though there was regrowth of cyst. The follow-up duration was defined as the period between the time of operation and the last MRI study.
To perform all statistical analyses, we used SPSS (version 21; IBM Corporation, Chicago, IL, USA). Differences with a pvalue <0.05 were considered statistically significant. The categorical variables were analyzed in terms of number of patients (%). The Kaplan-Meier survival curve with the log-rank test was calculated. We performed multivariable analysis using the binary logistic regression model to determine the hazard ratio (HR) of risk factors for recurrence.
Go to : 
Baseline characteristics are listed in Table 1. Twenty patients (62.5%) were women. Patients’ mean age was 40.8±14.9 years (median, 40.0; range, 29–75 years). The mean duration of folow-up was 62.3±48.6 months. Headache was the most common presenting symptom. Of the 16 patients with headache, 10 presented with acute-onset headache. Fourteen patients (43.8%) complained of visual impairment, and seven patients (21.9%) had nonspecific nausea or vomiting on presentation.
Basic characteristics
The ophthalmological test revealed that 18 patients (56.3%) had visual field defects. Endocrinological measurements revealed that 12 patients (37.5%) had one or more hormone deficiencies : ACTH for nine (28.1%), TSH in seven (21.9%), gonadotropin in six (18.8%), and GH in three (9.4%). Eleven patients (34.4%) had mild hyperprolactinemia, which might have been caused by the stalk effect; of these patients, one had amenorrhea, but the others had no clinical symptoms. Additionally, two patients (6.3%) had preoperative diabetes insipidus.
In the majority of cases, indications for surgery were visual impairment or worsening symptoms, such as headache. Of the 31 patients who had undergone surgery, reasons for cyst decompression include the following : 18 patients had visual field defect or compressive optic neuropathy; 13 patients had symptoms other than vision changes, which were related to RCCs such as headache and dizziness. Cysts were aspirated via microscopic (n=14) or endoscopic (n=16) endonasal approach in 30 patients (93.8%) and through a transcranial approach in two patients (6.3%). The endonasal approach was the first choice for procedures, but if the lesion was totally located in the suprasellar region, the transcranial approach was used so as not to damage the normal pituitary gland. In one patient, marked suprasellar growth of RCC necessitated an extended endoscopic endonasal transtuberculum approach. In most cases, the surgical procedure consisted of a simple fenestration for decompression, followed by partial resection of the cyst wall for biopsy. Intraoperative cerebrospinal fluid (CSF) leak was observed in 11 patients (34.4%).
After surgery, 21 patients (65.6%) showed no residual cysts on postoperative MRI and other 10 patients (31.3%) had small residual cysts. Of the 16 patients presenting with headache, 15 (93.8%) reported resolution of symptoms postoperatively. Of the 18 patients with preoperative visual field defect, 17 (94.4%) experienced visual improvement after surgery and one (5.6%) reported subjective stabilization of vision. With regard to endocrinological outcomes in all 32 patients, 11 (34.4%) exhibited hormonal deterioration, but the other 21 (65.6%) showed stabilization of or improvement in hormonal function. In 11 patients (34.4%), excluding those with preoperative diabetes insipidus, new-onset permanent diabetes insipidus developed postoperatively and the patients remained dependent on desmopressin. Central nervous system infection was observed in four patients (12.5%) : meningitis in three (9.4%) and pituitary abscess in one (3.1%). Postoperative intrasellar bleeding occurred in three patients (9.4%). In two patients (6.3%), CSF leaks developed postoperatively : one presented with meningitis, which resolved after conservative management with lumbar drainage and antibiotics; the other required reoperation. CSF leakage was not statistically associated with postoperative hormone deficiency (p=0.108), extent of resection (p=0.643) and cyst recurrence (p=0.459). We further measured the greatest cyst diameter on two-dimensional plane at the initial MRI and analyzed its association with surgical outcomes. The mean size of cysts preoperatively was 19.5±6.3 mm. However, the tumor diameter had no statistical association with extent of resection or cyst recurrence. Surgical outcomes in all patients are listed in Table 2.
Surgical outcomes in 32 patients with Rathke’s cleft cysts
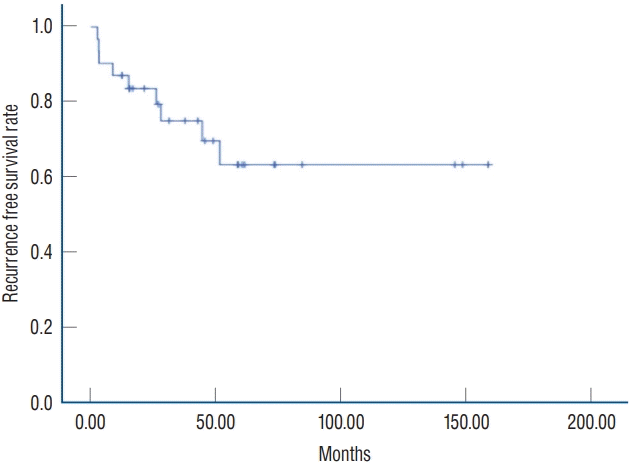
During the follow-up period, RCCs recurred in nine patients (28.1%), an average of 20.4 months after surgery. Of the nine patients, five patients among 21 patients with no residual cyst recurred and four patients among 10 patients with residual cyst progressed. Of these patients, three (33.3%) reported cyst-associated symptoms and underwent reoperation : two of these patients had aggravated visual field defect, and the third patient had headache, nausea, and vomiting. For all three patients, the postoperative clinical course was uneventful. The other six patients (66.7%) with recurrent RCCs received conservative management without surgery because five patients had no associated symptoms and one patient had shrinkage of the cyst based on MRI findings. Fig. 2 depicts the KaplanMeier estimated curve for recurrence free survival after surgery. The actuarial rates of recurrence-free survival at 2, 4, and 5 years after surgery were 83.6%, 69.7%, and 63.3%, respectively.
Univariable analysis revealed that no factor except postoperative endocrinological deterioration was associated with cyst recurrence; sex, age, postoperative residual cyst, and other factors showed no significant association with recurrence. Multivariable analysis also revealed that postoperative endocrinological deterioration was the only factor significantly associated with recurrence (p=0.028; HR, 6.800; Table 3).
Prognostic factors for recurrence
Go to : 
Preoperative symptoms associated with cysts improved after surgery in most cases : visual field defects and headache in 94.4% and 93.8% of patients, respectively. However, postoperative endocrinological deterioration and postoperative newonset diabetes insipidus each developed in 34.4% of all patients. Cysts recurred in nine patients (28.1%) and were associated with postoperative endocrinological deterioration.
RCCs typically arise within the sella between the anterior and posterior lobes of the pituitary gland [20]. Most often asymptomatic, RCCs have been found incidentally in 4% to 33% of autopsies [18,23,25]. However, these lesions can cause a mass effect on surrounding structures, such as the pituitary gland and optic chiasm, which can lead to headaches, pituitary dysfunction, and visual disturbance [1,3,23,25]. In patients with RCCs who develop progressive symptoms that are directly referable to the cysts and in those with visual field deficits or underlying laboratory evidence of endocrinopathy, surgical drainage of cysts remains the preferred treatment [25]. Headaches can also be considered as an indication for surgery. Several previous papers reported that headaches could be improved after surgery [8,16]. However, as headaches could be nonspecific and difficult to be considered to have association with RCCs, thorough and sufficient interview with patients and consideration were mandatory in all cases. So, in our study, those patients with large size RCCs enough to irritate meninges and with medication-refractory headaches were considered for surgical candidates.
Most of the patients in our cohort experienced resolution of or improvement in their symptoms postoperatively. All patients in our study experienced resolution of hyperprolactinemia, probably as a result of compression of the stalk. These results are similar to those in other series of patients with RCCs, in which headaches were alleviated and vision improved in 71–96% and 70–100% of affected patients, respectively [1-4,7,12,15,21]. However, as in other reports, anterior pituitary function improved in only 20–46% of patients after surgery [1,3,21]. Postoperative improvement in endocrinopathies may have been limited by capsular wall inflammation, surgical trauma, or prolonged compression of the gland [5,13,17]. We did not observe resolution of preoperative diabetes insipidus, in contrast to previous reports; in our cohort, however, a small number of patients had preexisting diabetes insipidus.
The most common surgical morbidities were endocrinopathies. Postoperative endocrinological aggravation and new-onset postoperative diabetes insipidus each developed in 34.4% of patients. Diabetes insipidus has been reported to be the most common postoperative complication in patients with RCCs [1], and radical resection of the cyst wall was associated with a higher rate of endocrinological morbidity [1,3,5,13]. However, the rate of postoperative diabetes insipidus was much higher in our study than in other series, although our surgical strategy was not radical cyst resection, but cyst fenestration for decompression. Moreover, new-onset pituitary dysfunction was rare in other series. There was no statistically significant factor for postoperative endocrine deterioration. We hypothesize that anterior pituitary dysfunction and diabetes insipidus that necessitate hormone replacement are much more common than expected and negatively affect quality of life. As previously mentioned, postoperative endocrinopathy may result from surgical trauma or cyst wall inflammation. Endocrine deterioration occurs as a result of cyst wall inflammation, which can give statistical significance to cyst recurrence. In view of these results, surgical indications of RCCs must be strict. No life-threatening complications occurred postoperatively. The incidences of surgery-related complications, such as hemorrhage, CSF leakage, and infection, were acceptable.
Approximately 28% of our cohort experienced a recurrence. Recurrence rates have varied widely, from 0% to >50% [3,5,9,12,13,19,21,24,25]. The variation in these outcomes might be attributable to the lack of a standardized definition of recurrence and to the variation in length of follow-up [13]. To date, no consensus has been reached about the possible predictors of RCC recurrence after surgery, including the extent of resection of the cyst wall, intraoperative alcohol instillation, sellar packing, reconstruction of the sellar floor, residual cyst shown on postoperative MRI, and inflammation and reactive squamous metaplasia revealed by histopathological examination [1-3,9,12,13,15]. Of interest was that not all recurrent cysts caused symptoms serious enough to warrant reoperation. Although imaging revealed evidence of RCC recurrence or progression in 28.1% of patients at an average of 20.4 months after surgery, only a third of these cysts were symptomatic and necessitated reoperation. This finding was consistent with that in previous studies : rates of cyst recurrence and reoperation were 26.6% and 9.2%, respectively [13]; 22% and 8%, respectively [2]; 30% and 0%, respectively [24]; and 50% and 3%, respectively [19]. Recurrent cysts that are apparent on follow-up MRI but cause no symptoms need not always be surgically resected, but they should be kept close observation.
Considering the aforementioned outcomes of RCC resection, minimal surgical manipulation would be reasonable for relieving symptoms and mitigating postoperative morbidity. Against our expectations, the extent of resection of the cyst wall was not associated with rate of recurrence in other studies [1,3,5]. According to Higgins et al. [5], recurrence rates did not differ significantly between patients who underwent no residual cyst (9%) and those who underwent decompression (17%; p=0.36), but the decompression cohort accounted for less complications postoperatively. Also, according to Higgins et al. [5], postoperative endocrinological deterioration was associated with cyst recurrence. Therefore, our strategy was to aspirate cyst contents and then perform gentle resection or biopsy of the cyst wall only when possible to cause minimal damage to the pituitary gland and thereby prevent new hormonal disorders. If the cyst wall is not movable during gentle dissection, it should not be removed. Of most importance is that strict criteria for surgical resection should be established. In our series, of the 406 patients who got “wait-and-see” management after being diagnosed with RCCs and followed up for more than 6 months, only one eventually underwent surgery. Conservative management is reasonable when the RCC does not change in size and in the absence of signs or symptoms of pituitary dysfunction in patients with smaller RCCs [22].
This study had several limitations. First, the retrospective design of this study may have resulted in selection bias. Second, although most patients followed our hospital protocol, postoperative follow-up or evaluations might not have been performed under the same conditions in all patients. Third, because of the relatively small sample size, no concrete criteria for surgical resection can be devised. Fourth, the follow-up periods were not long enough to evaluate the outcomes in patients with slowly growing lesions.
However, our study did show that patients who underwent surgical resection of RCCs had a much poorer prognosis than expected. In comparison with other sellar tumors, RCCs were accompanied by more endocrinological deterioration and recurred more often after surgery. Our result has shown that endocrinological aggravation seems to be the cause of recurrence, which in fact requires attention to interpretation. Because, as we mentioned above, even simple cyst fenestration for decompression itself could be a cause of cyst wall inflammation, which resulted in endocrinological deterioration. As a result, it might become a risk factor for cyst recurrence. Larger series with longer term follow-up are needed to clarify the postoperative course of patients who undergo RCC resection.
Go to : 
Our results showed that long-term results after cyst fenestration for decompression of RCCs were not that favorable than expected, although symptoms could be improved after surgery. Simple decompression itself could irritate normal pituitary gland and resulted in endocrinological aggravation, and might be considered as a risk factor for cyst recurrence. A decision of surgery for RCCs should be very careful. Further studies with large populations and longer follow-up are necessary to determine the treatment strategy.
Go to : 
Notes
Author contributions
Conceptualization : KH, JHH, CYK; Data curation : SHS, KH; Formal analysis : SHS, KH, SYJ; Funding acquisition : KH, CYK; Methodology : SHS, KH, SYJ; Project administration : KH, JHH, CYK; Visualization : SHS, KH; Writing - original draft : SHS, KH; Writing - review & editing : SHS, KH, SYJ, JHH, CYK
Go to : 
References
1. Aho CJ, Liu C, Zelman V, Couldwell WT, Weiss MH. Surgical outcomes in 118 patients with Rathke cleft cysts. J Neurosurg. 102:189–193. 2005.
2. Barkhoudarian G, Palejwala SK, Ansari S, Eisenberg AA, Huang X, Griffiths CF, et al. Rathke’s cleft cysts: a 6-year experience of surgery vs. observation with comparative volumetric analysis. Pituitary. 22:362–371. 2019.
3. Benveniste RJ, King WA, Walsh J, Lee JS, Naidich TP, Post KD. Surgery for Rathke cleft cysts: technical considerations and outcomes. J Neurosurg. 101:577–584. 2004.
4. Cabuk B, Selek A, Emengen A, Anik I, Canturk Z, Ceylan S. Clinicopathologic characteristics and endoscopic surgical outcomes of symptomatic Rathke’s cleft cysts. World Neurosurg. 132:e208–e216. 2019.
5. Higgins DM, Van Gompel JJ, Nippoldt TB, Meyer FB. Symptomatic Rathke cleft cysts: extent of resection and surgical complications. Neurosurg Focus. 31:E2. 2011.
6. Hwang K, Kim YH, Kim JH, Lee JH, Yang HK, Hwang JM, et al. The outcomes of conservatively observed asymptomatic nonfunctioning pituitary adenomas with optic nerve compression. J Neurosurg. 134:1808–1815. 2020.
7. Isono M, Kamida T, Kobayashi H, Shimomura T, Matsuyama J. Clinical features of symptomatic Rathke’s cleft cyst. Clin Neurol Neurosurg. 103:96–100. 2001.
8. Kim E. Symptomatic Rathke cleft cyst: clinical features and surgical outcomes. World Neurosurg. 78:527–534. 2012.
9. Kim JE, Kim JH, Kim OL, Paek SH, Kim DG, Chi JG, et al. Surgical treatment of symptomatic Rathke cleft cysts: clinical features and results with special attention to recurrence. J Neurosurg. 100:33–40. 2004.
10. Koutourousiou M, Grotenhuis A, Kontogeorgos G, Seretis A. Treatment of Rathke’s cleft cysts: experience at a single centre. J Clin Neurosci. 16:900–903. 2009.
11. Lee C, Park SH. Spontaneously regressed Rathke’s cleft cyst. J Korean Neurosurg Soc. 62:723–726. 2019.
12. Lillehei KO, Widdel L, Astete CA, Wierman ME, Kleinschmidt-DeMasters BK, Kerr JM. Transsphenoidal resection of 82 Rathke cleft cysts: limited value of alcohol cauterization in reducing recurrence rates. J Neurosurg. 114:310–317. 2011.
13. Lin M, Wedemeyer MA, Bradley D, Donoho DA, Fredrickson VL, Weiss MH, et al. Long-term surgical outcomes following transsphenoidal surgery in patients with Rathke’s cleft cysts. J Neurosurg. 130:831–837. 2018.
14. Mendelson ZS, Husain Q, Elmoursi S, Svider PF, Eloy JA, Liu JK. Rathke’s cleft cyst recurrence after transsphenoidal surgery: a meta-analysis of 1151 cases. J Clin Neurosci. 21:378–385. 2014.
15. Montaser AS, Catalino MP, Laws ER. Professor Rathke’s gift to neurosurgery: the cyst, its diagnosis, surgical management, and outcomes. Pituitary. 24:787–796. 2021.
16. Nishioka H, Haraoka J, Izawa H, Ikeda Y. Headaches associated with Rathke’s cleft cyst. Headache. 46:1580–1586. 2006.
17. Ogawa Y, Tominaga T, Ikeda H. Clinicopathological and endocrinological study of Rathke’s cleft cyst manifesting as hyponatremia. Neurol Med Chir (Tokyo). 47:58–63. discussion 63-64. 2007.
18. Osborn AG, Preece MT. Intracranial cysts: radiologic-pathologic correlation and imaging approach. Radiology. 239:650–664. 2006.
19. Petersson M, Berinder K, Eden Engström B, Tsatsaris E, Ekman B, Wahlberg J, et al. Natural history and surgical outcome of Rathke’s cleft cysts-a study from the Swedish Pituitary Registry. Clin Endocrinol (Oxf). 96:54–61. 2022.
20. Prabhu VC, Brown HG. The pathogenesis of craniopharyngiomas. Childs Nerv Syst. 21:622–627. 2005.
21. Ratha V, Patil S, Karmarkar VS, Shah NJ, Deopujari CE. Surgical management of Rathke cleft cysts. World Neurosurg. 107:276–284. 2017.
22. Sala E, Moore JM, Amorin A, Carosi G, Martinez H Jr, Harsh GR, et al. Natural history of Rathke’s cleft cysts: a retrospective analysis of a two centres experience. Clin Endocrinol (Oxf). 89:178–186. 2018.
23. Shatri J, Ahmetgjekaj I. Rathke’s cleft cyst or pituitary apoplexy: a case report and literature review. Open Access Maced J Med Sci. 6:544–547. 2018.
24. Yamada H, Ueda R, Ozawa H, Toda M. Long-term outcomes of endoscopic cyst fenestration for Rathke cleft cyst. World Neurosurg. 161:e282–e288. 2022.
25. Zada G. Rathke cleft cysts: a review of clinical and surgical management. Neurosurg Focus. 31:E1. 2011.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download