Abstract
A diagnosis of an intracranial aneurysm depends on the angiographic configuration and should be cautiously differentiated from aneurysm mimics. In cases of duplicate anterior choroidal arteries (AChAs), infundibular widening of the distal minor AChA can be an aneurysm mimic. If the minor AChA with a smaller diameter is obscured angiographically due to poor contrast filling, an associated infundibular widening beside the proximal large AChA can misinterpreted as a typical AChA aneurysm in angiograms. The authors report on two such cases of duplicate AChAs with infundibular widening presenting like a typical AChA aneurysm in angiograms. Surgical exploration revealed a perforating artery emitting from the dome of the saccular lesion, confirming infundibular widening of a duplicate AChA. No reparative procedure was applied to the infundibular widening in a 48-year-old man, while two vascular outpouchings from the infundibular widening were clipped preserving the duplicate AChA in a 55-year-old woman.
Among intracranial aneurysms, there is only a 2% to 5% incidence of a saccular aneurysm arising from the internal carotid artery (ICA) at the origin of the anterior choroidal artery (AChA) [6,31]. Yet, such aneurysms are well-known to neurosurgeons as they are particularly challenging for surgical or endovascular treatment due to the risk of inadvertent insufficiency of the AChA [2,7,8,16,17]. Typically, the AChA arises from the aneurysm base or the junction of the ICA and the aneurysm.
The diagnosis of a saccular aneurysm depends on the angiographic configuration and should be cautiously differentiated from aneurysm mimics, such as an arterial loop, arterial stump, or infundibular widening, to avoid unnecessary surgical or endovascular procedures and procedure-related complications. In cases of duplicate AChAs, infundibular widening of a distal minor AChA can be an aneurysm mimic. If the minor AChA with a small diameter is obscured angiographically due to poor contrast filling, an associated infundibular widening beside the proximal large AChA can be misinterpreted as a typical AChA aneurysm. Endovascular coiling of the infundibular widening can cause then serious AChA syndrome.
The authors report on two such cases of duplicate AChAs with infundibular widening presenting like a typical AChA aneurysm in angiograms. The two cases were identified among 415 surgical cases of an AChA aneurysm experienced by the two authors. The angiographic configurations and surgical findings are both presented.
A 48-year-old man presented with an incidental AChA aneurysm. Digital subtraction angiography (DSA) indicated a saccular (dome height, 4 mm; neck width, 2 mm) aneurysm with a high aspect ratio arising from the left ICA at the origin of the AChA (Fig. 1A). The AChA was identified at the proximal base of the aneurysm (Fig. 1B). The angiographic findings were identical to a typical AChA aneurysm.
A left supraorbital keyhole approach was performed by the first author to expose the lesion at the distal ICA. A saccular lesion was revealed in the lateral wall of the distal ICA with the prominent AChA arising at its proximal base. However, contrary to expectation, a perforating artery, which immediately divided into three branches, was observed arising from the dome of the saccular lesion (Fig. 1C and D). No irregularity or weak point requiring reparative surgical procedures was found in the wall of the saccular lesion. The diagnostic impression was infundibular widening of a minor duplicate AChA.
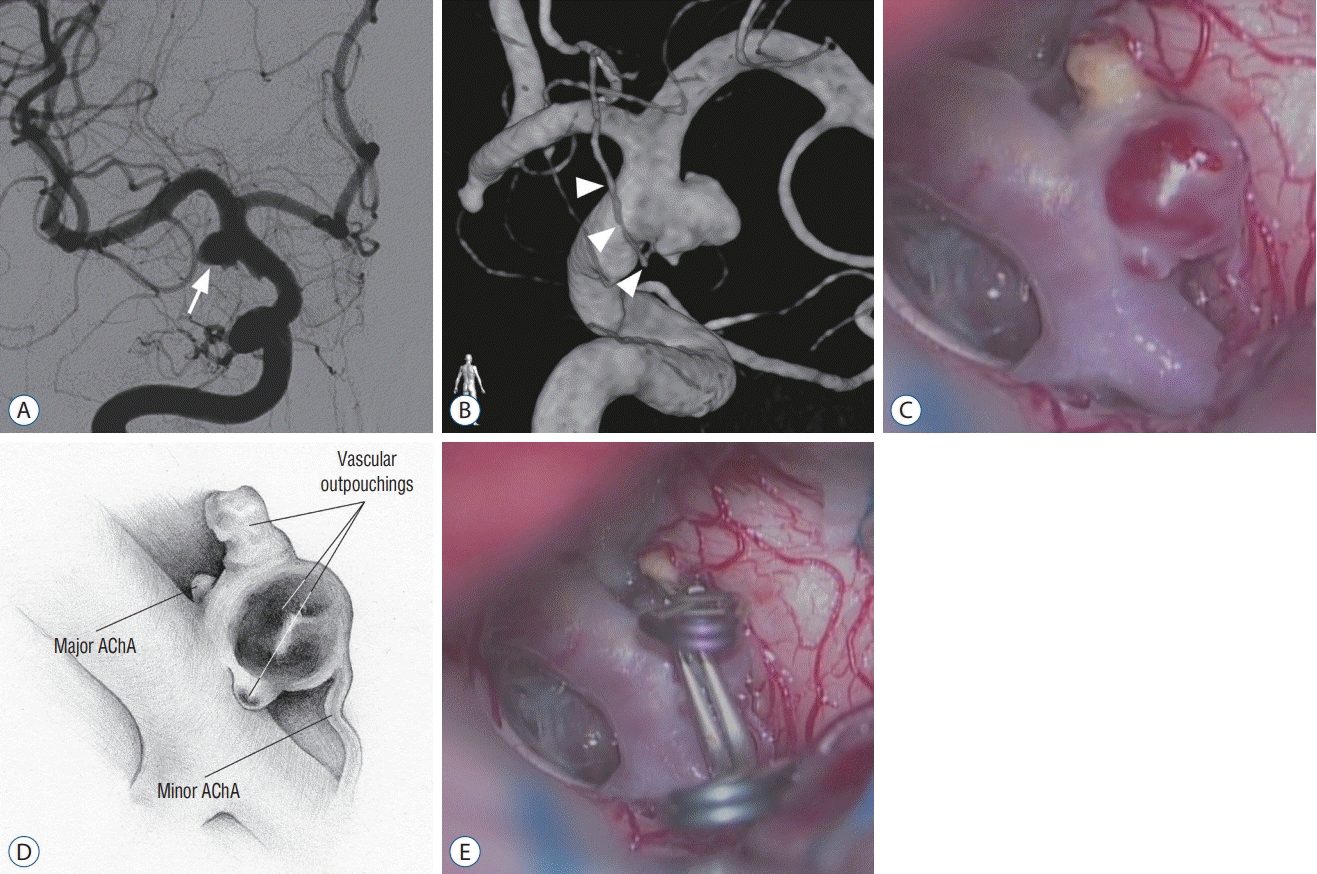
A 55-year-old woman was referred to the second author for treatment of an unruptured intracranial aneurysm. Angiographic examination yielded a diagnosis of a saccular AChA aneurysm. The right carotid angiography indicated a round saccular (dome height, 5 mm; neck width, 4 mm) aneurysm arising from the ICA at the origin of the AChA (Fig. 2A). The AChA was identified at the proximal base of the aneurysm (Fig. 2B).
In a right pterional craniotomy, a saccular lesion was revealed arising from the lateral wall of the distal ICA with the major AChA arising at its proximal base. However, a perforating artery was observed emitting from the dome of the saccular lesion (Fig. 2C and D). Temporary clipping of the perforating artery reduced the amplitude of the motor-evoked potentials (MEPs) of the contralateral lower extremity, confirming that the perforating artery was a duplicate AChA. The saccular lesion was interpreted as infundibular widening of a duplicate AChA. The infundibular widening had two vascular outpouchings : a degenerated yellow one and thin-walled red one. The two vascular outpouchings were both clipped, while the remaining infundibular widening and duplicate AChA were preserved (Fig. 2E). The patient awoke from the surgery with no neurological deficits.
To the authors’ knowledge, this is the first report of angiographically invisible duplicate AChA with infundibular widening mimicking a typical AChA aneurysm. Case 1 was among 180 surgical cases encountered by the first author, while case 2 was among 235 surgical cases encountered by the second author. Although the AChA usually arises from the ICA as a single branch, it can also originate as two or three branches [32]. Thus, associating the major AChA with infundibular widening of a thin, angiographically invisible, minor AChA can result in the misdiagnosis of a typical AChA aneurysm.
While infundibular widening is most commonly observed at the origin of the posterior communicating artery from the ICA [4,5,24,29], it can also occur at the origin of the AChA and ophthalmic artery [18,20], and rarely at the origins of the medial lenticulostriate artery from the A1 segment of the anterior cerebral artery, posterior cerebral artery (PCA), circumf lex branch from the P1 segment of the PCA, and superior cerebellar artery [14,21,30].
Careful angiographic evaluation to safeguard the identification of a vessel originating from the infundibular apex can provide a preoperative diagnosis of infundibular widening as long as the emitting vessel is visible. Volume-rendered threedimensional (3D) rotational angiography is superior to twodimensional (2D)-DSA in the detecting the relationship of an aneurysm to adjacent arteries [15]. Thus, when saccular bulging is detected at the distal ICA by 2D-DSA, 3D rotational angiography with volume rendering can provide useful information to distinguish infundibular widening from a true aneurysm [26]. In particular, grayscale modification of volumerendered 3D rotational angiography images can augment visualization of the emitting vessel as distinct from infundibuar widening [22].
Patients with infundibular widening of an angiographically invisible duplicate AChA can undergo surgical or endovascular procedures. In cases of surgical treatment, visual inspection under high microscopic magnification can reveal an angiographically invisible duplicate AChA and the vascular wall state of the infundibular widening. Thin-walled vascular areas with or without outpouchings in the infundibular widening can then be repaired using clipping or wrapping procedures. In contrast, if endovascular procedures had been performed for the reported cases, the patients would have been at high risk of arterial insufficiency for the angiographically invisible duplicate AChA and ensuing postprocedural neurological deficits.
Despite very few case series reports of endovascular-treated AChA aneurysms, significant concerns have been raised about AChA insufficiency due to thromboembolic occlusion of the AChA. Kang et al. [11] reported a 3.3% (three of 91 AChA aneurysms) case series incidence of procedure-related symptomatic AChA insufficiency, while Kim et al. [13] reported an incidence of 5.3% (two of 38 AChA aneurysms). Consequently, the current increasing trend of endovascular coiling for treating AChA aneurysms will expose patients with aneurysm mimics to a risk of procedure-related AChA insufficiency [11-13,23,25].
If endovascular coiling is performed in the case of an aneurysm mimic, such as the current cases, intraprocedural transcranial MEP monitoring could be useful for detecting an insufficiency of the angiographically invisible minor AChA in the anio suite. Changes in the MEP despite normal perfusion of the AChA during the coiling of an AChA aneurysm can suggest the possibility of an angiographically invisible minor AChA and should trigger the consideration of prompt salvage procedures, including coil removal.
Intraoperative MEP monitoring is widely used in the surgical clipping of cerebral aneurysms [3,9,27,28,33]. Moreover, intraprocedural transcranial MEP monitoring has been reported as useful during the endovascular coiling of intracranial aneurysms [10,19]. Nakagawa et al. [19] analyzed data from 164 consecutive patients who underwent endovascular coiling for intracranial aneurysms under transcranial MEP monitoring. Significant intraprocedural MEP changes occurred during seven of eight endovascular procedures associated with intraprocedural complications, facilitating immediate and salvage procedures. In a retrospective study by Ito et al. [10] of coiled unruptured AChA aeurysms with or without MEP monitoring, the volume embolization ratio of coiled aneurysms was significantly better for the patient group with MEP monitoring.
Although infundibular widening is considered as a benign anatomical variant resulting from incomplete regression of a fetal vessel, it can on rare occasions form a saccular aneurysm. Chen et al. [1] performed a comprehensive literature search and reviewed 15 case reports, including 16 cases describing aneurysm formation from infundibular widening at the posterior communicating artery origin. Thirteen of these patients (81.3%) were female. The median age at the time of diagnosis was 38 years (range, 23–55). Median time to aneurysmal progression after diagnosis was 7.5 years. Thirteen of these cases (81.3%) showed aneurysmal progression at or more than 5 years after the initial diagnosis. Trends in literature suggest that the female gender, presence of concurrent aneurysms, and hypertension may be associated with an increased risk of aneurysm formation from infundibular widening. Thus, patients with these risk factors may benefit from long-term follow up using magnetic resonance angiography or computed tomography angiography at approximately 5–7 years interval.
A diagnosis of an intracranial aneurysm depends on the angiographic configuration and should be cautiously differentiated from aneurysm mimics. In cases of duplicate AChAs, infundibular widening of a distal minor AChA can be an aneurysm mimic. In particular, if the minor AChA with a small diameter is obscured angiographically due to poor contrast filling, an associated infundibular widening beside the proximal large AChA cannot be differentiated from a saccular aneurysm.
Such aneurysm mimics require surgical treatment rather than endovascular coiling for exact diagnosis and an appropriate reparative procedure for the thin-walled lesion. Surgical exploration can reveal an AChA emitting from infundibular widening. Partial clipping or wrapping procedures can then be applied to the weak, thin-walled areas of the infundibular widening, while preserving the duplicate AChA.
Notes
Conflicts of interest
Jaechan Park has been editorial board of JKNS since May 2020. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
References
1. Chen CJ, Moosa S, Ding D, Raper DM, Burke RM, Lee CC, et al. Infundibular dilations of the posterior communicating arteries: pathogenesis, anatomical variants, aneurysm formation, and subarachnoid hemorrhage. J Neurointerv Surg. 8:791–795. 2016.
2. Cho MS, Kim MS, Chang CH, Kim SW, Kim SH, Choi BY. Analysis of clipinduced ischemic complication of anterior choroidal artery aneurysms. J Korean Neurosurg Soc. 43:131–134. 2008.
3. Chung J, Park W, Hong SH, Park JC, Ahn JS, Kwun BD, et al. Intraoperative use of transcranial motor/sensory evoked potential monitoring in the clipping of intracranial aneurysms: evaluation of false-positive and false-negative cases. J Neurosurg. 130:936–948. 2018.
4. Endo S, Furuichi S, Takaba M, Hirashima Y, Nishijima M, Takaku A. Clinical study of enlarged infundibular dilation of the origin of the posterior communicating artery. J Neurosurg. 83:421–425. 1995.
5. Epstein F, Ransohoff J, Budzilovich GN. The clinical significance of junctional dilatation of the posterior communicating artery. J Neurosurg. 33:529–531. 1970.
6. Flamm ES. Other aneurysms of the internal carotid artery in Wilkins RH, Rengachary SS (eds). Neurosurgery. New York: McGraw-Hill;1996. p. 2301.
7. Friedman JA, Pichelmann MA, Piepgras DG, Atkinson JL, Maher CO, Meyer FB, et al. Ischemic complications of surgery for anterior choroidal artery aneurysms. J Neurosurg. 94:565–572. 2001.
8. Furtado SV, Venkatesh PK, Hegde AS. Neurological complications and surgical outcome in patients with anterior choroidal segment aneurysms. Int J Neurosci. 120:291–297. 2010.
9. Irie T, Yoshitani K, Ohnishi Y, Shinzawa M, Miura N, Kusaka Y, et al. The efficacy of motor-evoked potentials on cerebral aneurysm surgery and new-onset postoperative motor deficits. J Neurosurg Anesthesiol. 22:247–251. 2010.
10. Ito A, Sato K, Niizuma K, Endo H, Matsumoto Y, Tominaga T. Intraoperative motor-evoked potential monitoring during coil embolization for anterior choroidal artery aneurysms. Neuroradiology. 64:1221–1229. 2022.
11. Kang HS, Kwon BJ, Kwon OK, Jung C, Kim JE, Oh CW, et al. Endovascular coil embolization of anterior choroidal artery aneurysms. Clinical article. J Neurosurg. 111:963–969. 2009.
12. Kim BM, Kim DI, Chung EC, Kim SY, Shin YS, Park SI, et al. Endovascular coil embolization for anterior choroidal artery aneurysms. Neuroradiology. 50:251–257. 2008.
13. Kim BM, Kim DI, Shin YS, Chung EC, Kim DJ, Suh SH, et al. Clinical outcome and ischemic complication after treatment of anterior choroidal artery aneurysm: comparison between surgical clipping and endovascular coiling. AJNR Am J Neuroradiol. 29:286–290. 2008.
14. Koike G, Seguchi K, Kyoshima K, Kobayashi S. Subarachnoid hemorrhage due to rupture of infundibular dilation of a circumflex branch of the posterior cerebral artery: case report. Neurosurgery. 34:1075–1077. 1994.
15. Kucukay F, Okten RS, Tekiner A, Dagli M, Gocek C, Bayar MA, et al. Three-dimensional volume rendering digital subtraction angiography in comparison with two-dimensional digital subtraction angiography and rotational angiography for detecting aneurysms and their morphological properties in patients with subarachnoid hemorrhage. Eur J Radiol. 81:2794–2800. 2012.
16. Lee YS, Park J. Anterior choroidal artery aneurysm surgery: ischemic complications and clinical outcomes revisited. J Korean Neurosurg Soc. 54:86–92. 2013.
17. Li J, Mukherjee R, Lan Z, Liu Y, He M. Microneurosurgical management of anterior choroidal artery aneurysms: a 16-year institutional experience of 102 patients. Neurol Res. 34:272–280. 2012.
18. Morris P. Practical Neuroangiography. ed 2. Philadelphia: Lippincott Williams & Wilkins;2007. p. p338–339.
19. Nakagawa I, Park H, Kotsugi M, Motoyama Y, Myochin K, Takeshima Y, et al. Diagnostic impact of monitoring transcranial motor-evoked potentials to prevent ischemic complications during endovascular treatment for intracranial aneurysms. Neurosurg Rev. 44:1493–1501. 2021.
20. Osborn AG. Diagnostic Cerebral Angiography. ed 2. Philadelphia: Lippincott Williams & Wilkins;1999. p. 272–273.
21. Papke K, Kuhl CK, Fruth M, Haupt C, Schlunz-Hendann M, Sauner D, et al. Intracranial aneurysms: role of multidetector CT angiography in diagnosis and endovascular therapy planning. Radiology. 244:532–540. 2007.
22. Park J, Kang DH. Infundibular widening mimicking anterior communicating artery aneurysm: report of 2 cases. J Neurosurg. 119:243–246. 2013.
23. Piotin M, Mounayer C, Spelle L, Williams MT, Moret J. Endovascular treatment of anterior choroidal artery aneurysms. AJNR Am J Neuroradiol. 25:314–318. 2004.
24. Saltzman GF. Infundibular widening of the posterior communicating artery studied by carotid angiography. Acta Radiol. 51:415–421. 1959.
25. Senturk C, Bandeira A, Bruneau M, Dewindt A, Balériaux D, De Witte O, et al. Endovascular treatment of anterior choroidal artery aneurysms. J Neuroradiol. 36:228–232. 2009.
26. Shi WY, Li YD, Li MH, Gu BX, Gu JP. Differential diagnosis of infundibular dilation versus a small aneurysm of the internal carotid artery: assessment by three-dimensional rotational angiography with volume rendering. Neurol Sci. 34:1065–1070. 2013.
27. Song J, Lang L, Zhu W, Gu Y, Xu B, Cai J, et al. Application of intraoperative motor evoked potential monitoring during giant internal carotid artery aneurysm surgery using prolonged temporary occlusion. Acta Neurochir (Wien). 157:1833–1840. 2015.
28. Suzuki K, Kodama N, Sasaki T, Matsumoto M, Konno Y, Sakuma J, et al. Intraoperative monitoring of blood flow insufficiency in the anterior choroidal artery during aneurysm surgery. J Neurosurg. 98:507–514. 2003.
29. Trasi S, Vincent LM, Zingesser LH. Development of aneurysm from infundibulum of posterior communicating artery with documentation of prior hemorrhage. AJNR Am J Neuroradiol. 2:368–370. 1981.
30. Wollschlaeger G, Wollschlaegerr PB. The circle of Willis in Newton TH, Potts DG (eds). Radiology of the Skull and Brain. St. Louis: CV Mosby;1974. Vol 2. p. 1171–1201.
31. Yasargil MG. Clinical considerations, surgery of intracranial aneurysms and results. New York: Thieme;1984. p. 99–108.
32. Yasargil MG, Yonas H, Gasser JC. Anterior choroidal artery aneurysms: their anatomy and surgical significance. Surg Neurol. 9:129–138. 1978.
33. Yeon JY, Seo DW, Hong SC, Kim JS. Transcranial motor evoked potential monitoring during the surgical clipping of unruptured intracranial aneurysms. J Neurol Sci. 293:29–34. 2010.
Fig. 1.
case 1. A : Two-dimensional digital subtraction angiography (2d-dSA) image suggesting a saccular aneurysm (arrow) arising from the internal carotid artery (IcA) at the origin of the anterior choroidal artery (AchA) (arrowheads). b : Volume-rendered three-dimensional (3d) rotational angiography image showing the origin of the AchA (arrowheads) at the base of the saccular lesion. c : Intraoperative photograph revealing a minor AchA emitting from infundibular widening with the major AchA at its base. d : Illustration corresponding to panel (c).
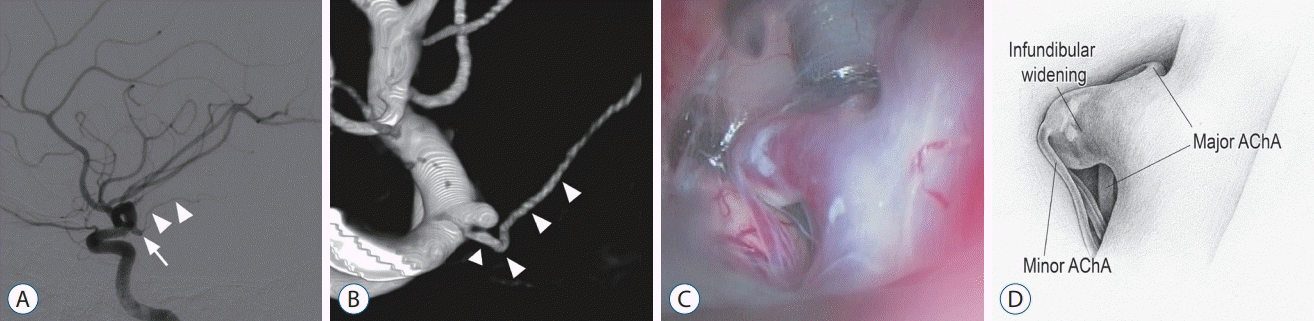
Fig. 2.
case 2. A : Two-dimensional digital subtraction angiography image showing an anterior choroidal artery (AchA) aneurysm (arrow). b : Volumerendered 3-dimensional rotational angiography image showing the origin of the AchA (arrowheads) at the proximal base of the saccular lesion. c : Intraoperative photograph revealing a minor AchA emitting from the dome of the saccular lesion with the major AchA at its base. Vascular outpouchings are seen in two areas. d : Illustration corresponding to panel (c). e : Intraoperative photograph showing the aneurysm clips repairing the aneurysmal changes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download