1. Demircay E, Civelek E, Cansever T, Kabatas S, Yilmaz C. Anatomic variations of the median nerve in the carpal tunnel: a brief review of the literature. Turk Neurosurg. 2011; 21:388–96.
2. Gelberman RH, Hergenroeder PT, Hargens AR, Lundborg GN, Akeson WH. The carpal tunnel syndrome: a study of carpal canal pressures. J Bone Joint Surg Am. 1981; 63:380–3.
3. Atroshi I, Gummesson C, Johnsson R, Ornstein E, Ranstam J, Rosen I. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999; 282:153–8.
4. Skie M, Zeiss J, Ebraheim NA, Jackson WT. Carpal tunnel changes and median nerve compression during wrist flexion and extension seen by magnetic resonance imaging. J Hand Surg Am. 1990; 15:934–9.
5. Uchiyama S, Itsubo T, Nakamura K, Kato H, Yasutomi T, Momose T. Current concepts of carpal tunnel syndrome: pathophysiology, treatment, and evaluation. J Orthop Sci. 2010; 15:1–13.
6. Boya H, Ozcan O, Ozteki N HH. Long-term complications of open carpal tunnel release. Muscle Nerve. 2008; 38:1443–6.
7. Kluge W, Simpson RG, Nicol AC. Late complications after open carpal tunnel decompression. J Hand Surg Br. 1996; 21:205–7.
8. Hui AC, Wong S, Leung CH, Tong P, Mok V, Poon D, et al. A randomized controlled trial of surgery vs steroid injection for carpal tunnel syndrome. Neurology. 2005; 64:2074–8.
9. Marshall S, Tardif G, Ashworth N. Local corticosteroid injection for carpal tunnel syndrome. Cochrane Database Syst Rev. 2007; (2):CD001554.
10. Chern TC, Wu KC, Huang LW, Shao CJ, Wu TT, Kuo LC, et al. A cadaveric and preliminary clinical study of ultrasonographically assisted percutaneous carpal tunnel release. Ultrasound Med Biol. 2014; 40:1819–26.
11. McShane JM, Slaff S, Gold JE, Nazarian LN. Sonographically guided percutaneous needle release of the carpal tunnel for treatment of carpal tunnel syndrome: preliminary report. J Ultrasound Med. 2012; 31:1341–9.
12. Rojo-Manaute JM, Capa-Grasa A, Chana-Rodriguez F, Perez-Mananes R, Rodriguez-Maruri G, Sanz-Ruiz P, et al. Ultra-minimally invasive ultrasound-guided carpal tunnel release: a randomized clinical trial. J Ultrasound Med. 2016; 35:1149–57.
13. Nakamichi K, Tachibana S. Distance between the median nerve and ulnar neurovascular bundle: clinical significance with ultrasonographically assisted carpal tunnel release. J Hand Surg Am. 1998; 23:870–4.
14. Buncke G, McCormack B, Bodor M. Ultrasound-guided carpal tunnel release using the manos CTR system. Microsurgery. 2013; 33:362–6.
15. Stevens JC. AAEM Minimonograph #26: the electrodiagnosis of carpal tunnel syndrome. Muscle Nerve. 1997; 20:1477–86.
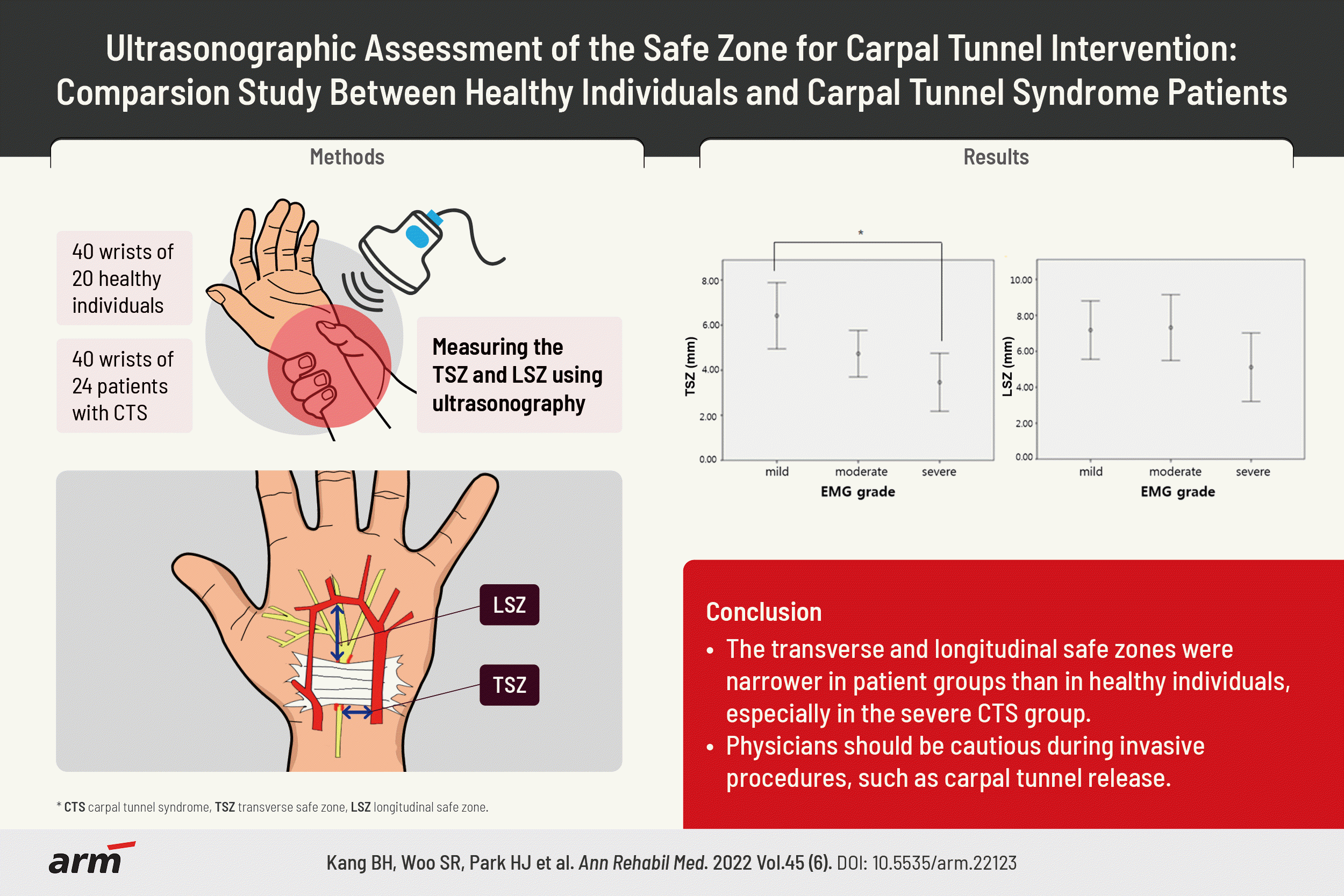
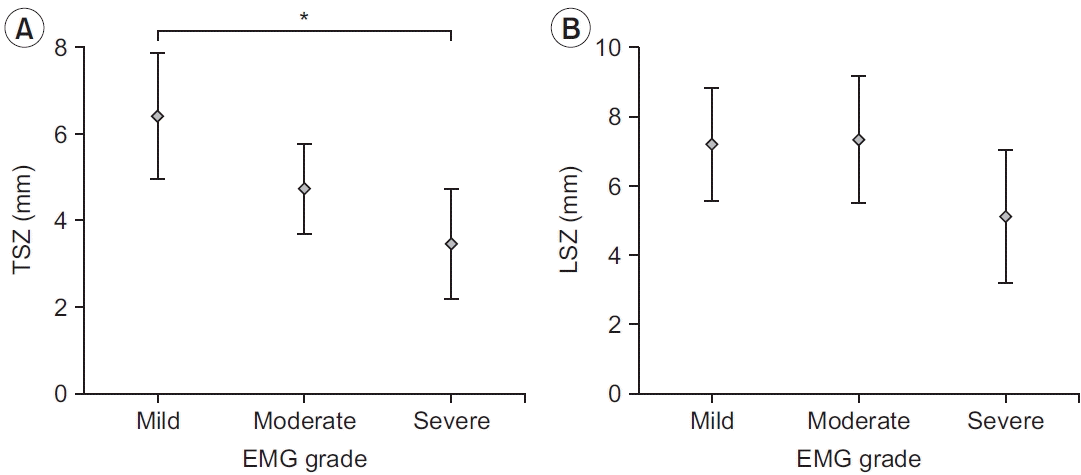
16. Lee HJ, Kwon HK. Electrophysiologic classification of severity of carpal tunnel syndrome. J Korean Assoc EMG-Electrodiagn Med. 2004; 6:1–3.
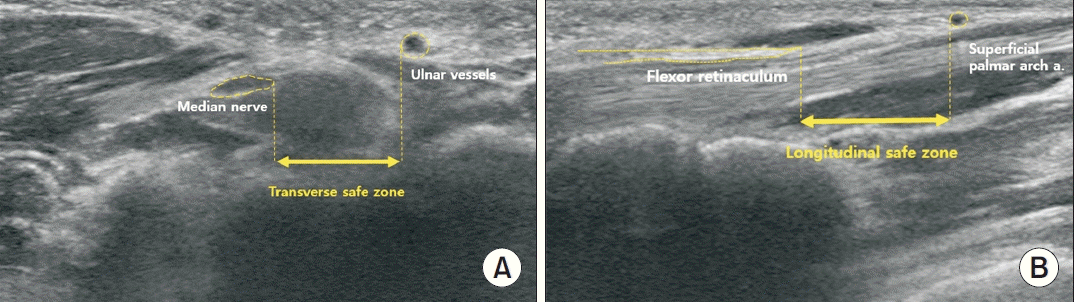
17. Ajayi NO, Naidoo N, Lazarus L, Satyapal KS. Determination of the median nerve safe-zone in the carpal tunnel using the distal forearm bony prominences. Folia Morphol (Warsz). 2014; 73:409–13.
18. Omokawa S, Tanaka Y, Ryu J, Suzuki J, Kish VL. Anatomy of the ulnar artery as it relates to the transverse carpal ligament. J Hand Surg Am. 2002; 27:101–4.
19. Rotman MB, Manske PR. Anatomic relationships of an endoscopic carpal tunnel device to surrounding structures. J Hand Surg Am. 1993; 18:442–50.
20. Olave E, Del Sol M, Gabriellp C, Mandiola E, Rodrigues CF. Biometric study of the relationships between palmar neurovas cular str uctures, the flexor retinaculum and the distal wrist crease. J Anat. 2001; 198(Pt 6):737–41.
21. Bayrak IK, Bayrak AO, Tilki HE, Nural MS, Sunter T. Ultrasonography in carpal tunnel syndrome: comparison with electrophysiological stage and motor unit number estimate. Muscle Nerve. 2007; 35:344–8.
22. Lee CH, Kim TK, Yoon ES, Dhong ES. Correlation of high-resolution ultrasonographic findings with the clinical symptoms and electrodiagnostic data in carpal tunnel syndrome. Ann Plast Surg. 2005; 54:20–3.
23. Padua L, Pazzaglia C, Caliandro P, Granata G, Foschini M, Briani C, et al. Carpal tunnel syndrome: ultrasound, neurophysiology, clinical and patient-oriented assessment. Clin Neurophysiol. 2008; 119:2064–9.
24. Ustun N, Tok F, Yagz AE, Kizil N, Korkmaz I, Karazincir S, et al. Ultrasound-guided vs. blind steroid injections in carpal tunnel syndrome: a single-blind randomized prospective study. Am J Phys Med Rehabil. 2013; 92:999–1004.
25. Kim PT, Lee HJ, Kim TG, Jeon IH. Current approaches for carpal tunnel syndrome. Clin Orthop Surg. 2014; 6:253–7.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download