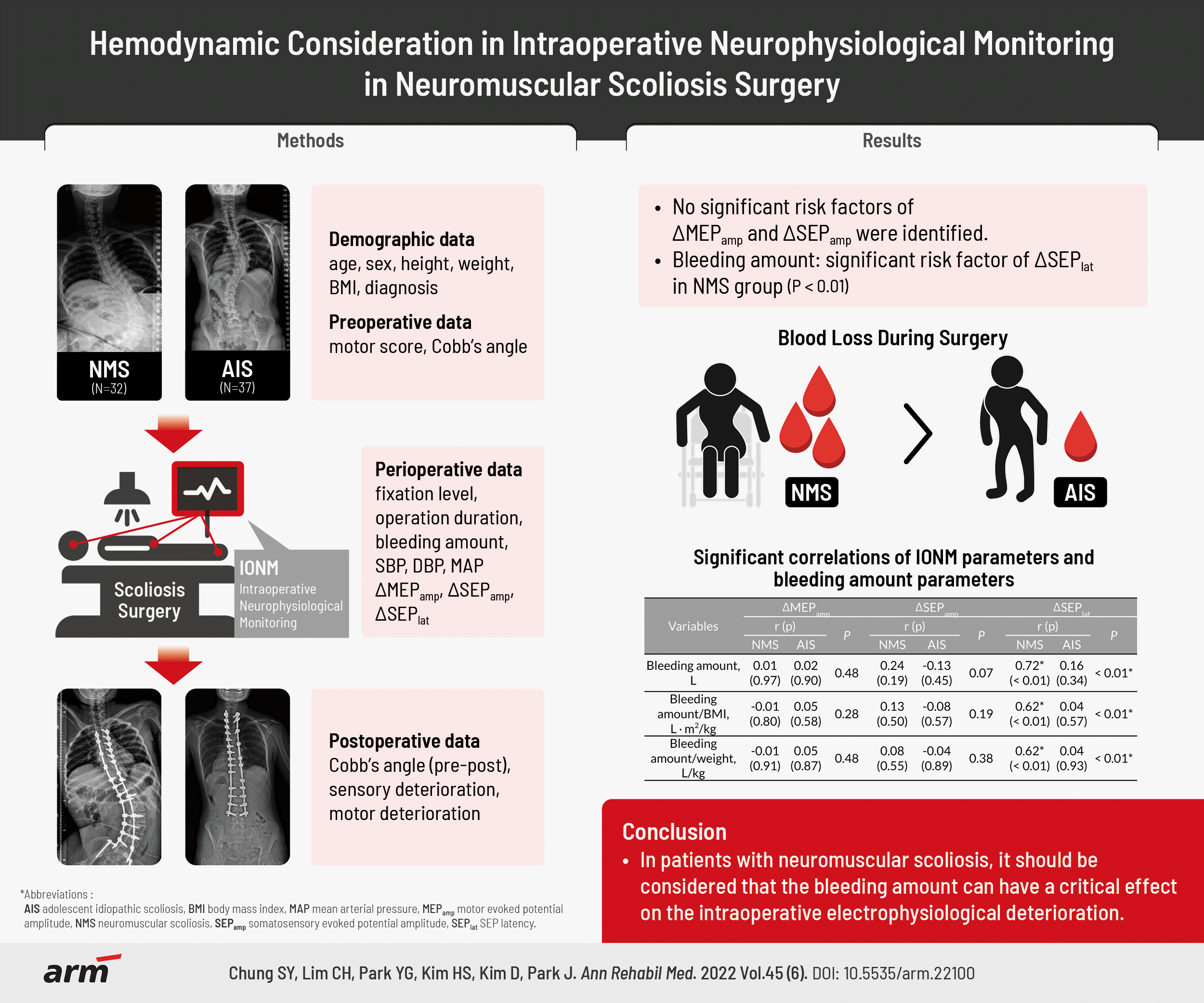
Abstract
Objective
Methods
Results
Notes
Conceptualization: Park JY. Methodology: Park JY. Formal analysis: Chung SY, Lim CH, Kim DW. Project administration: Lim CH, Park YG, Park JY. Visualization: Chung SY, Lim CH, Park JY. Writing – original draft: Chung SY, Lim CH. Writing – review and editing: Park JY. Approval of final manuscript: all authors.
REFERENCES
Table 1.
| Characteristic | Sub-category | NMS (n=32) | AIS (n=37) | p-value | |
|---|---|---|---|---|---|
| Demographic | Age (yr) | 15.3 (10–22) | 15.5 (9–23) | 0.78 | |
| Sex | <0.01* | ||||
| Male | 25 (78.1) | 5 (13.5) | |||
| Female | 7 (21.9) | 32 (86.5) | |||
| Diagnosis | |||||
| Adolescent idiopathic scoliosis | - | 37 (100) | |||
| Muscular dystrophya) | 19 (59.4) | - | |||
| Spinal muscular atrophy | 4 (12.5) | - | |||
| Othersb) | 9 (28.1) | - | |||
| Height (cm) | 148.8 (118.0–172.0) | 160.4 (124.0–188.4) | <0.01* | ||
| Weight (kg) | 38.8 (18.0–80.8) | 53.8 (24.5–122.8) | <0.01* | ||
| BMI (kg/m2) | 17.4 (9.2–31.7) | 20.6 (15.3–34.6) | <0.01* | ||
| Preoperative | Preoperative motor score | 23.8±13.6 | 50.0±0.0 | <0.01* | |
| Cobb’spre (°) | 64.7±16.6 | 50.1±7.0 | <0.01* | ||
| Perioperative | Fixation level | 15.7±1.2 | 11.7±2.4 | <0.01* | |
| Operation duration (hr) | 8.0±1.9 | 6.8±1.6 | <0.01* | ||
| Body temperature (°C) | 36.0±0.5 | 36.2±0.3 | 0.21 | ||
| Hemodynamics | |||||
| Bleeding amount (L) | 2.59±1.58 | 1.67±0.88 | <0.01* | ||
| SBPmin (mmHg) | 76.6±8.4 | 82.4±5.6 | <0.01* | ||
| DBPmin (mmHg) | 39.2±4.8 | 41.1±4.7 | 0.11 | ||
| MAPmin (mmHg) | 51.7±5.3 | 54.8±4.3 | <0.01* | ||
| Use of anesthetics | 0.53 | ||||
| Remifentanil+propofol | 10 (31.3) | 9 (24.3) | |||
| Remifentanil+propofol+midazolam | 22 (68.7) | 28 (75.7) | |||
| Use of muscle relaxant for intubation | |||||
| Rocuronium bromide (mg) | 55.5±15.2 | 54.1±12.7 | 0.68 | ||
| Use of vasopressor | 21 (65.6) | 11 (29.7) | <0.01* | ||
| Transfusion (L) | 1.56±1.23 | 0.76±0.56 | <0.01* | ||
| IONM parameters | |||||
| ΔMEPamp >80% reduction | 12 (37.5) | 6 (16.2) | 0.04* | ||
| ΔSEPamp >50% reduction | 3 (9.4) | 5 (13.5) | 1.00 | ||
| ΔSEPlat >10% prolongation | 0 (0) | 0 (0) | 1.00 | ||
| ΔMEPamp | 21.5±53.7 | 12.5±32.8 | 0.42 | ||
| ΔSEPamp | 35.9±18.2 | 35.0±17.8 | 0.82 | ||
| ΔSEPlat | 5.3±7.5 | 3.2±2.5 | 0.11 | ||
| Postoperative | ΔCobb’s (°) | 39.0±17.0 | 39.8±6.5 | 0.82 | |
| Postoperative sensory deterioration | 0 (0) | 0 (0) | 1.00 | ||
| Postoperative motor deterioration | 0 (0) | 0 (0) | 1.00 | ||
Values are presented as mean (range) or number (%) or mean±standard deviation.
AIS, adolescent idiopathic scoliosis; BMI, body mass index; Cobb’spre, preoperative Cobb’s angle; DBPmin, minimum diastolic blood pressure; MAPmin, minimum mean arterial pressure; NMS, neuromuscular scoliosis; SBPmin, minimum systolic blood pressure; ΔCobb’s, corrected Cobb’s angle via scoliosis surgery; ΔMEPamp, maximum amplitude decrement percentage of motor evoked potentials compared with baseline values; ΔSEPamp, maximum amplitude decrement percentage of somatosensory evoked potentials compared with baseline values; ΔSEPlat, maximum latency prolongation percentage of somatosensory evoked potentials compared with baseline values.
a) Duchenne muscular dystrophy (n=12), progressive muscular dystrophy (n=5), myotonic muscular dystrophy (n=1), and limb girdle muscular dystrophy (n=1).
Table 2.
|
ΔMEPamp |
ΔSEPamp |
ΔSEPlat |
||||
|---|---|---|---|---|---|---|
| NMS | AIS | NMS | AIS | NMS | AIS | |
| Age | 0.86 | 0.49 | 0.80 | 0.28 | 0.50 | 0.35 |
| Height | 0.87 | 0.96 | 0.14 | 0.67 | 0.23 | 0.86 |
| Weight | 0.46 | 0.96 | 0.75 | 0.59 | 0.20 | 0.26 |
| Preoperative motor score | 0.77 | 0.22 | 0.44 | 0.12 | 0.28 | 0.25 |
| Cobb’spre | 0.61 | 0.43 | 1.00 | 0.95 | 0.82 | 0.39 |
| Fixation level | 0.91 | 0.26 | 0.38 | 0.97 | 0.70 | 0.17 |
| Operation duration | 0.73 | 0.16 | 0.58 | 0.28 | 0.11 | 0.27 |
| Bleeding amount | 1.00 | 0.28 | 0.33 | 0.18 | <0.01* | 0.41 |
| SBPmin | 0.12 | 0.69 | 0.52 | 0.11 | 0.86 | 0.47 |
| DBPmin | 0.28 | 0.28 | 0.10 | 0.60 | 0.20 | 0.62 |
| MAPmin | 0.44 | 0.26 | 0.73 | 0.20 | 0.32 | 0.23 |
IONM, intraoperative neurophysiological monitoring; ΔMEPamp, maximum amplitude decrement percentage of motor evoked potentials compared with baseline values; ΔSEPamp, maximum amplitude decrement percentage of somatosensory evoked potentials compared with baseline values; ΔSEPlat, maximum latency prolongation percentage of somatosensory evoked potentials compared with baseline values; NMS, neuromuscular scoliosis; AIS, adolescent idiopathic scoliosis; Cobb’spre, preoperative Cobb’s angle; SBPmin, minimum systolic blood pressure; DBPmin, minimum diastolic blood pressure; MAPmin, minimum mean arterial blood pressure.
Table 3.
| Variable | NMS | AIS | p-value (intergroup difference) | Ratio of NMS/AIS |
|---|---|---|---|---|
| Bleeding amount (L) | 2.59±1.58 | 1.67±0.88 | <0.01* | 1.56 |
| Bleeding amount/BMI (L∙m2/kg) | 0.17±0.14 | 0.08±0.04 | <0.01* | 2.13 |
| Bleeding amount/weight (L/kg) | 0.08±0.07 | 0.03±0.02 | <0.01* | 2.62 |
Table 4.
| Variable |
ΔMEPamp |
ΔSEPamp |
ΔSEPlat |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
NMS |
AIS |
p-value (intergroup difference) |
NMS |
AIS |
p-value (intergroup difference) |
NMS |
AIS |
p-value (intergroup difference) | |||||||
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | ||||
| Bleeding amount (L) | 0.01 | 0.97 | 0.02 | 0.90 | 0.48 | 0.24 | 0.19 | -0.13 | 0.45 | 0.07 | 0.72 | <0.01* | 0.16 | 0.34 | <0.01* |
| Bleeding amount/BMI (L∙m2/kg) | -0.01 | 0.80 | 0.05 | 0.58 | 0.28 | 0.13 | 0.50 | -0.08 | 0.57 | 0.19 | 0.62 | <0.01* | 0.04 | 0.57 | <0.01* |
| Bleeding amount/ weight (L/kg) | -0.01 | 0.91 | 0.05 | 0.87 | 0.48 | 0.08 | 0.55 | -0.04 | 0.89 | 0.38 | 0.62 | <0.01* | 0.04 | 0.93 | <0.01* |
NMS, neuromuscular scoliosis; AIS, adolescent idiopathic scoliosis; BMI, body mass index; ΔMEPamp, maximum amplitude decrement percentage of motor evoked potentials compared with baseline values; ΔSEPamp, maximum amplitude decrement of somatosensory evoked potentials compared with baseline values; ΔSEPlat, maximum latency delay of somatosensory evoked potential compared with baseline values.
Table 5.
| Variable |
MAPmin |
SBPmin |
DBPmin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
NMS |
AIS |
NMS |
AIS |
NMS |
AIS |
|||||||
| r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | r | p-value | |
| Bleeding amount (L) | -0.48 | 0.01* | -0.17 | 0.32 | -0.45 | 0.01* | -0.11 | 0.53 | -0.39 | 0.03* | -0.17 | 0.32 |
| Bleeding amount/BMI (L∙m2/kg) | -0.64 | <0.01* | -0.20 | 0.24 | -0.59 | <0.01* | -0.17 | 0.33 | -0.53 | <0.01* | -0.17 | 0.30 |
| Bleeding amount/weight (L/kg) | -0.69 | <0.01* | -0.24 | 0.15 | -0.64 | <0.01* | -0.21 | 0.22 | -0.56 | <0.01* | -0.21 | 0.22 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download