1. Ziegler-Graham K, MacKenzie EJ, Ephraim PL, Travison TG, Brookmeyer R. Estimating the prevalence of limb loss in the United States: 2005 to 2050. Arch Phys Med Rehabil. 2008; 89:422–9.
2. Miller WC, Speechley M, Deathe B. The prevalence and risk factors of falling and fear of falling among lower extremity amputees. Arch Phys Med Rehabil. 2001; 82:1031–7.
3. Langford J, Dillon MP, Granger CL, Barr C. Physical activity participation amongst individuals with lower limb amputation. Disabil Rehabil. 2019; 41:1063–70.
4. Young AJ, Simon AM, Hargrove LJ. A training method for locomotion mode prediction using powered lower limb prostheses. IEEE Trans Neural Syst Rehabil Eng. 2014; 22:671–7.
5. Isakov E, Burger H, Gregoric M, Marincek C. Stump length as related to atrophy and strength of the thigh muscles in trans-tibial amputees. Prosthet Orthot Int. 1996; 20:96–100.
6. Genin JJ, Bastien GJ, Franck B, Detrembleur C, Willems PA. Effect of speed on the energy cost of walking in unilateral traumatic lower limb amputees. Eur J Appl Physiol. 2008; 103:655–63.
7. Goktepe AS, Cakir B, Yilmaz B, Yazicioglu K. Energy expenditure of walking with prostheses: comparison of three amputation levels. Prosthet Orthot Int. 2010; 34:31–6.
8. Waters RL, Mulroy S. The energy expenditure of normal and pathologic gait. Gait Posture. 1999; 9:207–31.
9. Gottschalk FA, Stills M. The biomechanics of transfemoral amputation. Prosthet Orthot Int. 1994; 18:12–7.
10. Thiele B, James U, Stalberg E. Neurophysiological studies on muscle function in the stump of above-knee amputees. Scand J Rehabil Med. 1973; 5:67–70.
11. Rutkowska-Kucharska A, Kowal M, Winiarski S. Relationship between asymmetry of gait and muscle torque in patients after unilateral transfemoral amputation. Appl Bionics Biomech. 2018; 2018:5190816.
12. Kowal M, Rutkowska-Kucharska A. Muscle torque of the hip joint flexors and extensors in physically active and inactive amputees. Biomed Hum Kinet. 2014; 6:63–8.
13. Erskine RM, Jones DA, Maganaris CN, Degens H. In vivo specific tension of the human quadriceps femoris muscle. Eur J Appl Physiol. 2009; 106:827–38.
14. Akagi R, Suzuki M, Kawaguchi E, Miyamoto N, Yamada Y, Ema R. Muscle size-strength relationship including ultrasonographic echo intensity and voluntary activation level of a muscle group. Arch Gerontol Geriatr. 2018; 75:185–90.
15. Putz C, Block J, Gantz S, Heitzmann DW, Dreher T, Lehner B, et al. Structural changes in the thigh muscles following trans-femoral amputation. Eur J Orthop Surg Traumatol. 2017; 27:829–35.
16. Leijendekkers RA, Marra MA, Ploegmakers MJ, Van Hinte G, Frolke JP, Van De Meent H, et al. Magnetic-resonance-imaging-based three-dimensional muscle reconstruction of hip abductor muscle volume in a person with a transfemoral bone-anchored prosthesis: a feasibility study. Physiother Theory Pract. 2019; 35:495–504.
17. Jaegers SM, Arendzen JH, de Jongh HJ. Changes in hip muscles after above-knee amputation. Clin Orthop Relat Res. 1995; (319):276–84.
18. Unver B, Baris RH, Yuksel E, Cekmece S, Kalkan S, Karatosun V. Reliability of 4-meter and 10-meter walk tests after lower extremity surgery. Disabil Rehabil. 2017; 39:2572–6.
19. Major MJ, Fatone S, Roth EJ. Validity and reliability of the Berg Balance Scale for community-dwelling persons with lower-limb amputation. Arch Phys Med Rehabil. 2013; 94:2194–202.
20. Donovan K, Lord SE, McNaughton HK, Weatherall M. Mobility beyond the clinic: the effect of environment on gait and its measurement in community-ambulant stroke survivors. Clin Rehabil. 2008; 22:556–63.
21. Lusardi MM, Pellecchia GL, Schulman M. Functional performance in community living older adults. J Geriatr Phys Ther. 2003; 26:14–22.
22. American College of Sports Medicine. ACSM’s resource manual for guidelines for exercise testing and prescription. 5th ed. Baltimore, MD: Lippincott Williams & Wilkins;2006.
23. Downs S, Marquez J, Chiarelli P. Normative scores on the Berg Balance Scale decline after age 70 years in healthy community-dwelling people: a systematic review. J Physiother. 2014; 60:85–9.
24. Batten HR, McPhail SM, Mandrusiak AM, Varghese PN, Kuys SS. Gait speed as an indicator of prosthetic walking potential following lower limb amputation. Prosthet Orthot Int. 2019; 43:196–203.
25. Wentink EC, Prinsen EC, Rietman JS, Veltink PH. Comparison of muscle activity patterns of transfemoral amputees and control subjects during walking. J Neuroeng Rehabil. 2013; 10:87.
26. Hong JH, Mun MS. Relationship between socket pressure and EMG of two muscles in trans-femoral stumps during gait. Prosthet Orthot Int. 2005; 29:59–72.
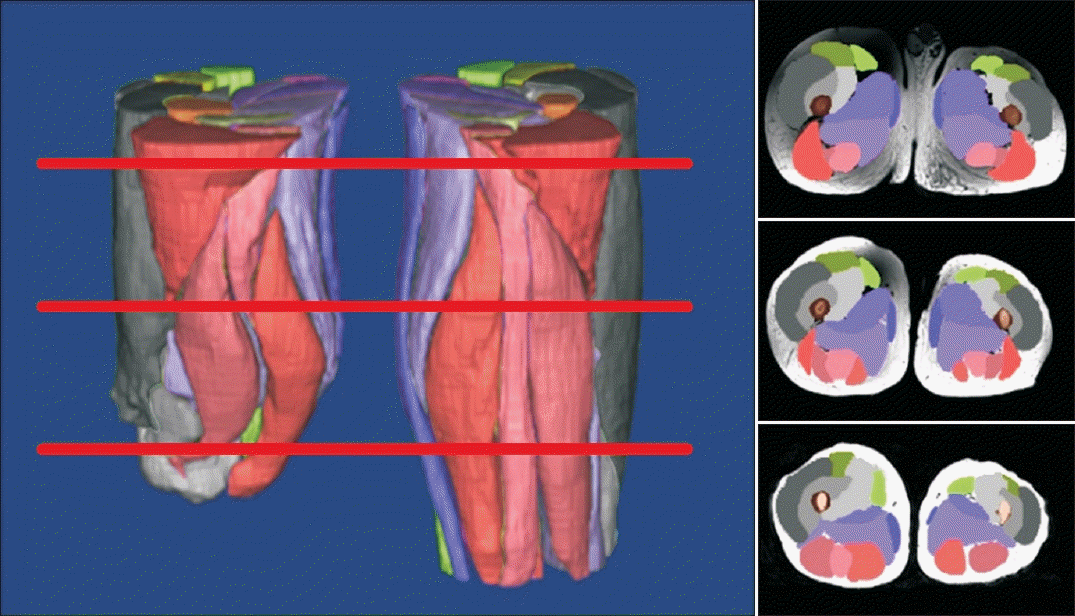




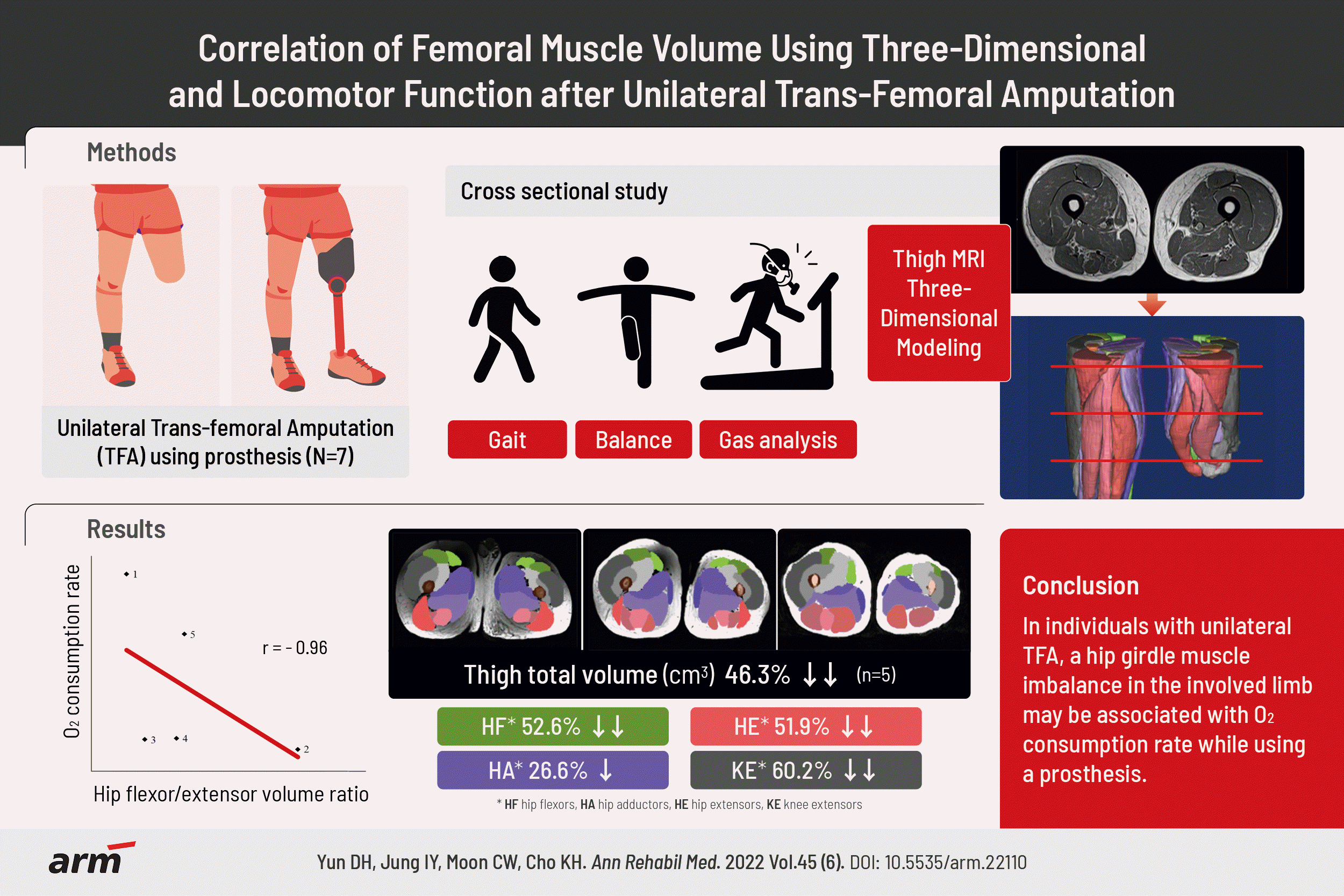
 PDF
PDF Citation
Citation Print
Print



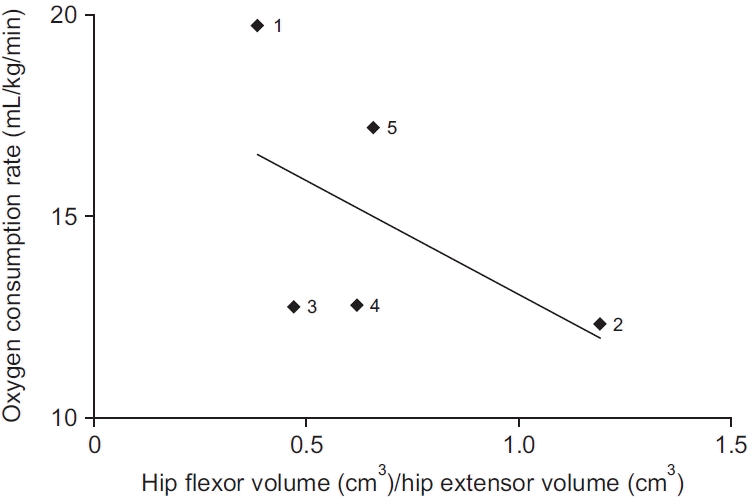
 XML Download
XML Download