1. Sneyd JR, Rigby-Jones AE. New drugs and technologies, intravenous anaesthesia is on the move (again). Br J Anaesth. 2010; 105:246–54.

2. Oka S, Satomi H, Sekino R, Taguchi K, Kajiwara M, Oi Y, et al. Sedation outcomes for remimazolam, a new benzodiazepine. J Oral Sci. 2021; 63:209–11.
3. Claeys MA, Gepts E, Camu F. Haemodynamic changes during anaesthesia induced and maintained with propofol. Br J Anaesth. 1988; 60:3–9.
4. Ryu T, Kim BJ, Woo SJ, Lee SY, Lim JA, Kwak SG, et al. Retrospective analysis of risk factors of hypotensive bradycardic events during shoulder arthroscopic surgery under interscalene blockade in the sitting position. Korean J Anesthesiol. 2020; 73:542–9.
5. Tan CH, Onsiong MK. Pain on injection of propofol. Anaesthesia. 1998; 53:468–76.
6. Ebert TJ. Sympathetic and hemodynamic effects of moderate and deep sedation with propofol in humans. Anesthesiology. 2005; 103:20–4.

7. Kanto JH. Midazolam: the first water-soluble benzodiazepine. Pharmacology, pharmacokinetics and efficacy in insomnia and anesthesia. Pharmacotherapy. 1985; 5:138–55.
8. Nordt SP, Clark RF. Midazolam: a review of therapeutic uses and toxicity. J Emerg Med. 1997; 15:357–65.

9. Borkett KM, Riff DS, Schwartz HI, Winkle PJ, Pambianco DJ, Lees JP, et al. A phase IIa, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015; 120:771–80.
10. Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012; 115:274–83.
11. Kilpatrick GJ, McIntyre MS, Cox RF, Stafford JA, Pacofsky GJ, Lovell GG, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007; 107:60–6.
12. Rex DK, Bhandari R, Desta T, DeMicco MP, Schaeffer C, Etzkorn K, et al. A phase III study evaluating the efficacy and safety of remimazolam (CNS 7056) compared with placebo and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2018; 88:427–37.e6.

13. Kim SH, Fechner J. Remimazolam - current knowledge on a new intravenous benzodiazepine anesthetic agent. Korean J Anesthesiol. 2022; 75:307–15.
14. Zhang X, Li S, Liu J. Efficacy and safety of remimazolam besylate versus propofol during hysteroscopy: single-centre randomized controlled trial. BMC Anesthesiol. 2021; 21:156.

15. Doi M, Morita K, Takeda J, Sakamoto A, Yamakage M, Suzuki T. Efficacy and safety of remimazolam versus propofol for general anesthesia: a multicenter, single-blind, randomized, parallel-group, phase IIb/III trial. J Anesth. 2020; 34:543–53.

16. Doi M, Hirata N, Suzuki T, Morisaki H, Morimatsu H, Sakamoto A. Safety and efficacy of remimazolam in induction and maintenance of general anesthesia in high-risk surgical patients (ASA Class III): results of a multicenter, randomized, double-blind, parallel-group comparative trial. J Anesth. 2020; 34:491–501.
17. Dai G, Pei L, Duan F, Liao M, Zhang Y, Zhu M, et al. Safety and efficacy of remimazolam compared with propofol in induction of general anesthesia. Minerva Anestesiol. 2021; 87:1073–9.

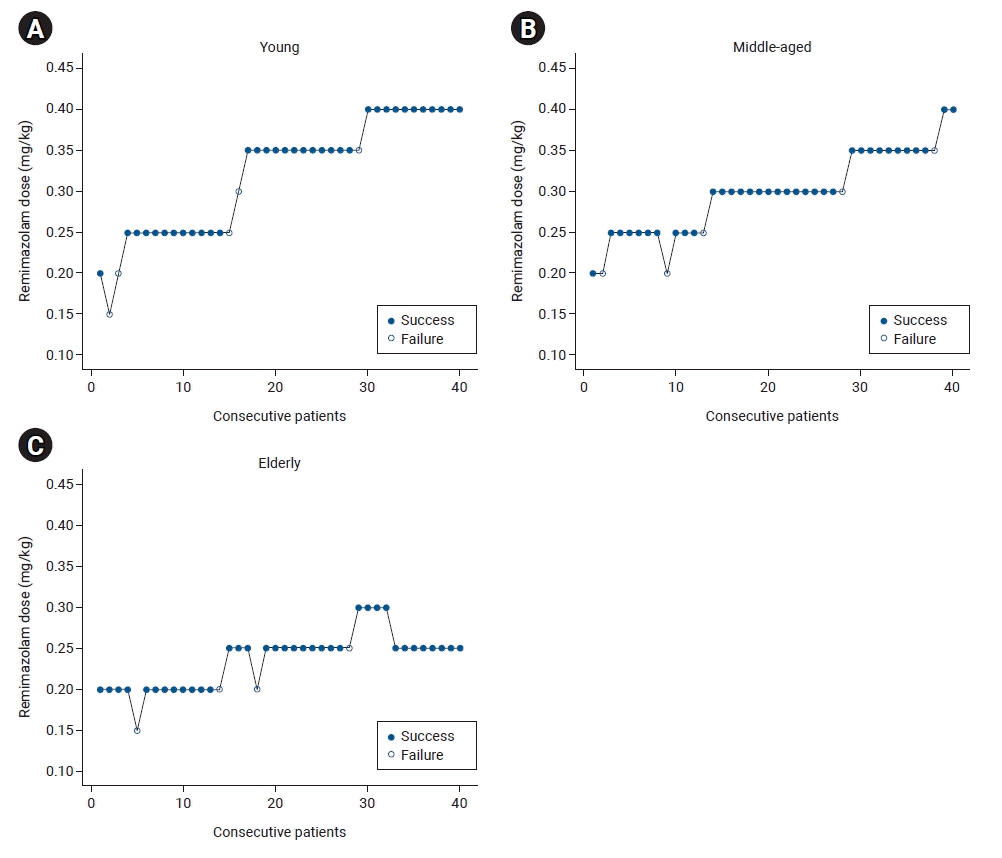
18. Durham SD, Flournoy N, Rosenberger WF. A random walk rule for phase I clinical trials. Biometrics. 1997; 53:745–60.

19. Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a precis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007; 107:144–52.
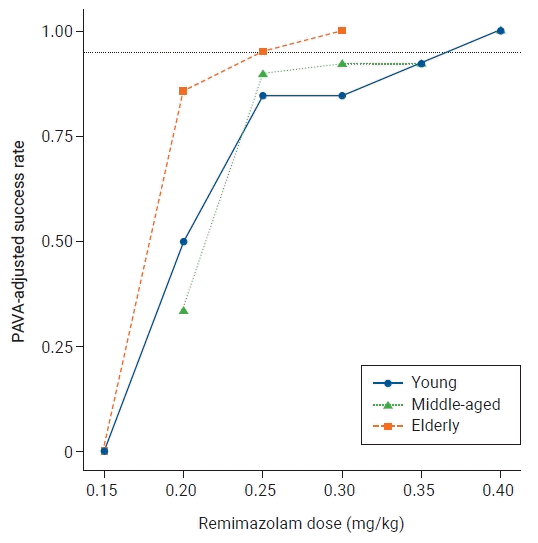
20. Stylianou M, Flournoy N. Dose finding using the biased coin up-and-down design and isotonic regression. Biometrics. 2002; 58:171–7.

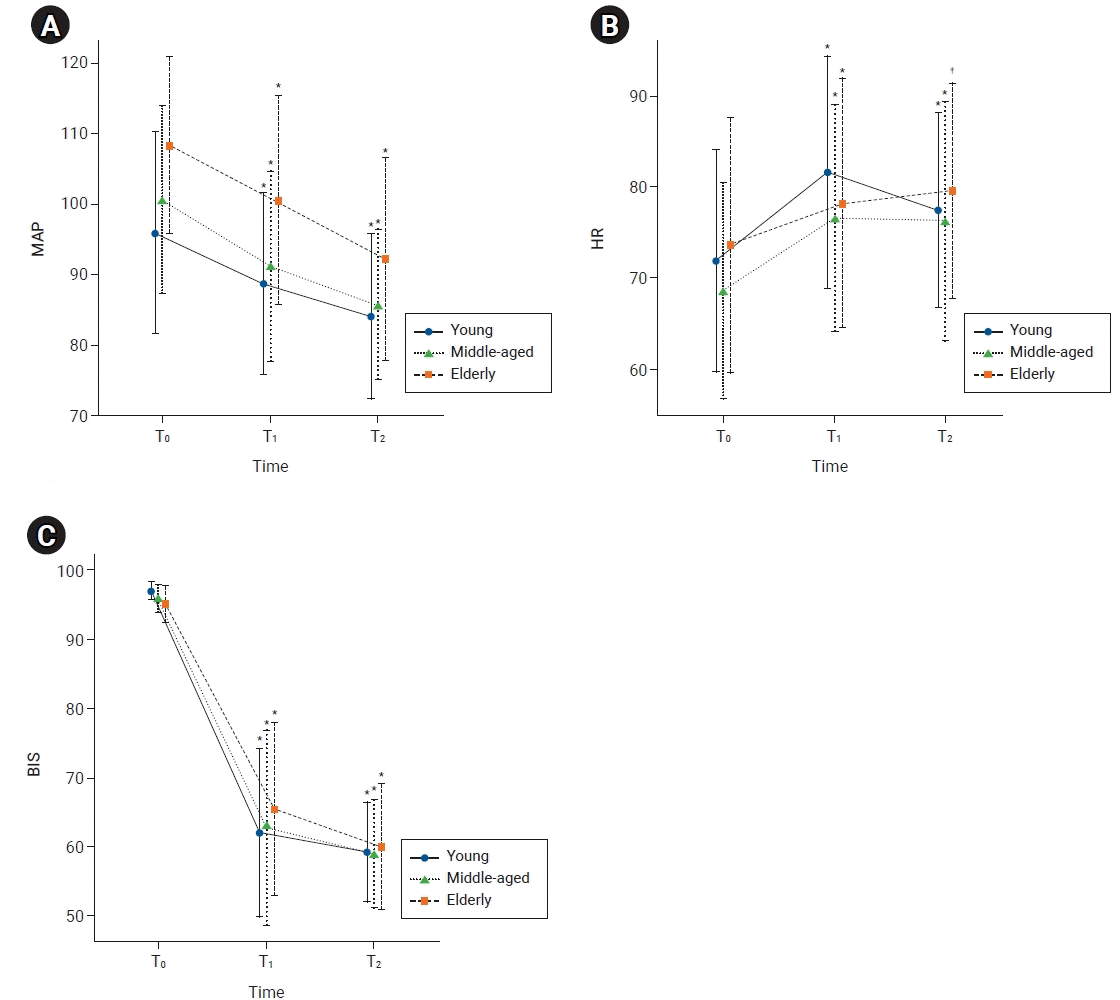
21. Krejcie TC, Avram MJ. What determines anesthetic induction dose? It’s the front-end kinetics, doctor! Anesth Analg. 1999; 89:541–4.

22. Zhang S, Wang J, Ran R, Peng Y, Xiao Y. Efficacy and safety of remimazolam tosylate in hysteroscopy: a randomized, single-blind, parallel controlled trial. J Clin Pharm Ther. 2022; 47:55–60.

23. Shi F, Chen Y, Li H, Zhang Y, Zhao T. Efficacy and safety of remimazolam tosilate versus propofol for general anesthesia in cirrhotic patients undergoing endoscopic variceal ligation. Int J Gen Med. 2022; 15:583–91.

24. Gorges M, Zhou G, Brant R, Ansermino JM. Sequential allocation trial design in anesthesia: an introduction to methods, modeling, and clinical applications. Paediatr Anaesth. 2017; 27:240–7.
25. Olutoye OA, Yu X, Govindan K, Tjia IM, East DL, Spearman R, et al. The effect of obesity on the ED(95) of propofol for loss of consciousness in children and adolescents. Anesth Analg. 2012; 115:147–53.

26. Park YH, Chung EJ, Lee JH, Kim JT, Kim CS, Kim HS. Determination of the 95% effective dose of remifentanil for the prevention of coughing during extubation in children undergoing tonsillectomy (with or without adenoidectomy). Paediatr Anaesth. 2015; 25:567–72.

27. Dosani M, McCormack J, Reimer E, Brant R, Dumont G, Lim J, et al. Slower administration of propofol preserves adequate respiration in children. Paediatr Anaesth. 2010; 20:1001–8.

28. Wu B, Shan J, Zhou Q, Wang L. Determination of the ED95 of a single bolus dose of dexmedetomidine for adequate sedation in obese or nonobese children and adolescents. Br J Anaesth. 2021; 126:684–91.
29. Li S, Liu H, Zhang J, Liu Y, Yu Q, Sun M, et al. The 95% effective dose of intranasal dexmedetomidine sedation for pulmonary function testing in children aged 1-3 years: a biased coin design up-and-down sequential method. J Clin Anesth. 2020; 63:109746.
30. Doi M. Remimazolam. J Jpn Soc Clin Anesth. 2014; 34:860–6.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download