1. Veeravagu A, Chen YR, Ludwig C, Rincon F, Maltenfort M, Jallo J, et al. Acute lung injury in patients with subarachnoid hemorrhage: a nationwide inpatient sample study. World Neurosurg. 2014; 82:e235–41.

2. Han DW. Brain and lung: dangerous crosstalk. Korean J Anesthesiol. 2017; 70:116–7.

3. Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011; 147:728–41.
4. Ravanan P, Srikumar IF, Talwar P. Autophagy: the spotlight for cellular stress responses. Life Sci. 2017; 188:53–67.

5. Deretic V, Saitoh T, Akira S. Autophagy in infection, inflammation and immunity. Nat Rev Immunol. 2013; 13:722–37.

6. Patel AS, Morse D, Choi AM. Regulation and functional significance of autophagy in respiratory cell biology and disease. Am J Respir Cell Mol Biol. 2013; 48:1–9.
7. Vishnupriya S, Priya Dharshini LC, Sakthivel KM, Rasmi RR. Autophagy markers as mediators of lung injury-implication for therapeutic intervention. Life Sci. 2020; 260:118308.

8. Bao N, Tang B. Organ-protective effects and the underlying mechanism of dexmedetomidine. Mediators Inflamm. 2020; 2020:6136105.

9. Hasanin A, Taha K, Abdelhamid B, Abougabal A, Elsayad M, Refaie A, et al. Evaluation of the effects of dexmedetomidine infusion on oxygenation and lung mechanics in morbidly obese patients with restrictive lung disease. BMC Anesthesiol. 2018; 18:104.

10. Lee SH, Kim N, Lee CY, Ban MG, Oh YJ. Effects of dexmedetomidine on oxygenation and lung mechanics in patients with moderate chronic obstructive pulmonary disease undergoing lung cancer surgery: a randomised double-blinded trial. Eur J Anaesthesiol. 2016; 33:275–82.
11. Zhang W, Zhang J. Dexmedetomidine preconditioning protects against lung injury induced by ischemia-reperfusion through inhibition of autophagy. Exp Ther Med. 2017; 14:973–80.
12. Ding D, Xu S, Zhang H, Zhao W, Zhang X, Jiang Y, et al. 3-Methyladenine and dexmedetomidine reverse lipopolysaccharide-induced acute lung injury through the inhibition of inflammation and autophagy. Exp Ther Med. 2018; 15:3516–22.

13. Li ZB, Li GC, Qin J. Dexmedetomidine attenuates lung injury in toxic shock rats by inhibiting inflammation and autophagy. Arch Med Res. 2021; 52:277–83.

14. Zhu C, Zhou Q, Luo C, Chen Y. Dexmedetomidine protects against oxygen-glucose deprivation-induced injury through inducing astrocytes autophagy via TSC2/mTOR pathway. Neuromolecular Med. 2020; 22:210–7.

15. Yu T, Liu D, Gao M, Yang P, Zhang M, Song F, et al. Dexmedetomidine prevents septic myocardial dysfunction in rats via activation of α7nAChR and PI3K/Akt- mediated autophagy. Biomed Pharmacother. 2019; 120:109231.

16. Sehba FA. Rat endovascular perforation model. Transl Stroke Res. 2014; 5:660–8.

17. Sugawara T, Ayer R, Jadhav V, Zhang JH. A new grading system evaluating bleeding scale in filament perforation subarachnoid hemorrhage rat model. J Neurosci Methods. 2008; 167:327–34.

18. Jeon H, Ai J, Sabri M, Tariq A, Shang X, Chen G, et al. Neurological and neurobehavioral assessment of experimental subarachnoid hemorrhage. BMC Neurosci. 2009; 10:103.

19. Chen J, Qian C, Duan H, Cao S, Yu X, Li J, et al. Melatonin attenuates neurogenic pulmonary edema via the regulation of inflammation and apoptosis after subarachnoid hemorrhage in rats. J Pineal Res. 2015; 59:469–77.

20. Levine B, Mizushima N, Virgin HW. Autophagy in immunity and inflammation. Nature. 2011; 469:323–35.

21. Tang D, Kang R, Coyne CB, Zeh HJ, Lotze MT. PAMPs and DAMPs: signal 0s that spur autophagy and immunity. Immunol Rev. 2012; 249:158–75.
22. Hu R, Xu H, Jiang H, Zhang Y, Sun Y. The role of TLR4 in the pathogenesis of indirect acute lung injury. Front Biosci (Landmark Ed). 2013; 18:1244–55.

23. Sun Q, Wu W, Hu YC, Li H, Zhang D, Li S, et al. Early release of high-mobility group box 1 (HMGB1) from neurons in experimental subarachnoid hemorrhage in vivo and in vitro. J Neuroinflammation. 2014; 11:106.

24. Zhang K, Huang Q, Deng S, Yang Y, Li J, Wang S. Mechanisms of TLR4-mediated autophagy and nitroxidative stress. Front Cell Infect Microbiol. 2021; 11:766590.

25. Kuang M, Cen Y, Qin R, Shang S, Zhai Z, Liu C, et al. Artesunate attenuates pro-inflammatory cytokine release from macrophages by inhibiting TLR4-mediated autophagic activation via the TRAF6-Beclin1-PI3KC3 pathway. Cell Physiol Biochem. 2018; 47:475–88.

26. Qian M, Fang X, Wang X. Autophagy and inflammation. Clin Transl Med. 2017; 6:24.

27. Liu X, Cao H, Li J, Wang B, Zhang P, Dong Zhang X, et al. Autophagy induced by DAMPs facilitates the inflammation response in lungs undergoing ischemia-reperfusion injury through promoting TRAF6 ubiquitination. Cell Death Differ. 2017; 24:683–93.

28. Zhang X, Zheng J, Yan Y, Ruan Z, Su Y, Wang J, et al. Angiotensin-converting enzyme 2 regulates autophagy in acute lung injury through AMPK/mTOR signaling. Arch Biochem Biophys. 2019; 672:108061.
29. Zeng M, Sang W, Chen S, Chen R, Zhang H, Xue F, et al. 4-PBA inhibits LPS-induced inflammation through regulating ER stress and autophagy in acute lung injury models. Toxicol Lett. 2017; 271:26–37.

30. Xu X, Zhi T, Chao H, Jiang K, Liu Y, Bao Z, et al. Corrigendum to "ERK1/2/mTOR/Stat3 pathway-mediated autophagy alleviates traumatic brain injury-induced acute lung injury" [Biochim. Biophys. Acta 1864/5PA(2018) 1663-1674]. Biochim Biophys Acta Mol Basis Dis. 2018; 1864:2214.

31. Santacruz CA, Vincent JL, Bader A, Rincon-Gutierrez LA, Dominguez-Curell C, Communi D, et al. Association of cerebrospinal fluid protein biomarkers with outcomes in patients with traumatic and non-traumatic acute brain injury: systematic review of the literature. Crit Care. 2021; 25:278.
32. Beitler JR, Malhotra A, Thompson BT. Ventilator-induced lung injury. Clin Chest Med. 2016; 37:633–46.

33. Zhang Y, Liu G, Dull RO, Schwartz DE, Hu G. Autophagy in pulmonary macrophages mediates lung inflammatory injury via NLRP3 inflammasome activation during mechanical ventilation. Am J Physiol Lung Cell Mol Physiol. 2014; 307:L173–85.
34. López-Alonso I, Aguirre A, González-Lopez A, Fernández ÁF, Amado-Rodríguez L, Astudillo A, et al. Impairment of autophagy decreases ventilator-induced lung injury by blockade of the NF-κB pathway. Am J Physiol Lung Cell Mol Physiol. 2013; 304:L844–52.

35. Jing R, Hu ZK, Lin F, He S, Zhang SS, Ge WY, et al. Mitophagy-mediated mtDNA release aggravates stretching-induced inflammation and lung epithelial cell injury via the TLR9/MyD88/NF-κB pathway. Front Cell Dev Biol. 2020; 8:819.

36. Wang Y, Wang C, Zhang D, Wang H, Bo L, Deng X. Dexmedetomidine protects against traumatic brain injury-induced acute lung injury in mice. Med Sci Monit. 2018; 24:4961–7.

37. Wu Y, Liu Y, Huang H, Zhu Y, Zhang Y, Lu F, et al. Dexmedetomidine inhibits inflammatory reaction in lung tissues of septic rats by suppressing TLR4/NF-κB pathway. Mediators Inflamm. 2013; 2013:562154.
38. Liu JR, Yuki K, Baek C, Han XH, Soriano SG. Dexmedetomidine-induced neuroapoptosis is dependent on its cumulative dose. Anesth Analg. 2016; 123:1008–17.

39. Wei Q, Chen J, Xiao F, Tu Y, Zhong Y, Xie Y. High-dose dexmedetomidine promotes apoptosis in fetal rat hippocampal neurons. Drug Des Devel Ther. 2021; 15:2433–44.

40. Hanci V, Yurdakan G, Yurtlu S, Turan IÖ, Sipahi EY. Protective effect of dexmedetomidine in a rat model of α-naphthylthiourea-induced acute lung injury. J Surg Res. 2012; 178:424–30.

41. Song Y, Lim BJ, Kim DH, Ju JW, Han DW. Effect of dexmedetomidine on cerebral vasospasm and associated biomarkers in a rat subarachnoid hemorrhage model. J Neurosurg Anesthesiol. 2019; 31:342–9.

42. Ahuja N, Andres-Hernando A, Altmann C, Bhargava R, Bacalja J, Webb RG, et al. Circulating IL-6 mediates lung injury via CXCL1 production after acute kidney injury in mice. Am J Physiol Renal Physiol. 2012; 303:F864–72.

43. Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-gamma: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004; 75:163–89.
44. Malaguarnera M, Di Fazio I, Laurino A, Pistone G, Restuccia S, Trovato BA. Decrease of interferon gamma serum levels in patients with chronic hepatitis C. Biomed Pharmacother. 1997; 51:391–6.

45. Boelens PG, Houdijk AP, Fonk JC, Puyana JC, Haarman HJ, von Blomberg-van der Flier ME, et al. Glutamine-enriched enteral nutrition increases in vitro interferon-gamma production but does not influence the in vivo specific antibody response to KLH after severe trauma. A prospective, double blind, randomized clinical study. Clin Nutr. 2004; 23:391–400.

46. Hu PJ, Pittet JF, Kerby JD, Bosarge PL, Wagener BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol. 2017; 313:L1–15.

47. Chen G, Wu D, Guo W, Cao Y, Huang D, Wang H, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020; 130:2620–9.

48. Hu ZJ, Xu J, Yin JM, Li L, Hou W, Zhang LL, et al. Lower circulating interferon-gamma is a risk factor for lung fibrosis in COVID-19 patients. Front Immunol. 2020; 11:585647.

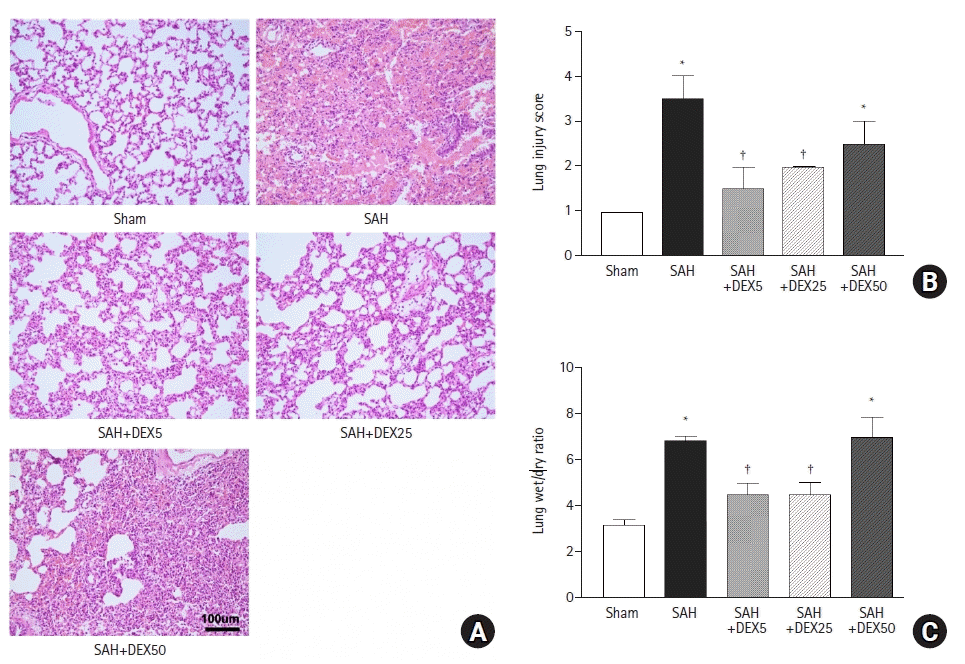
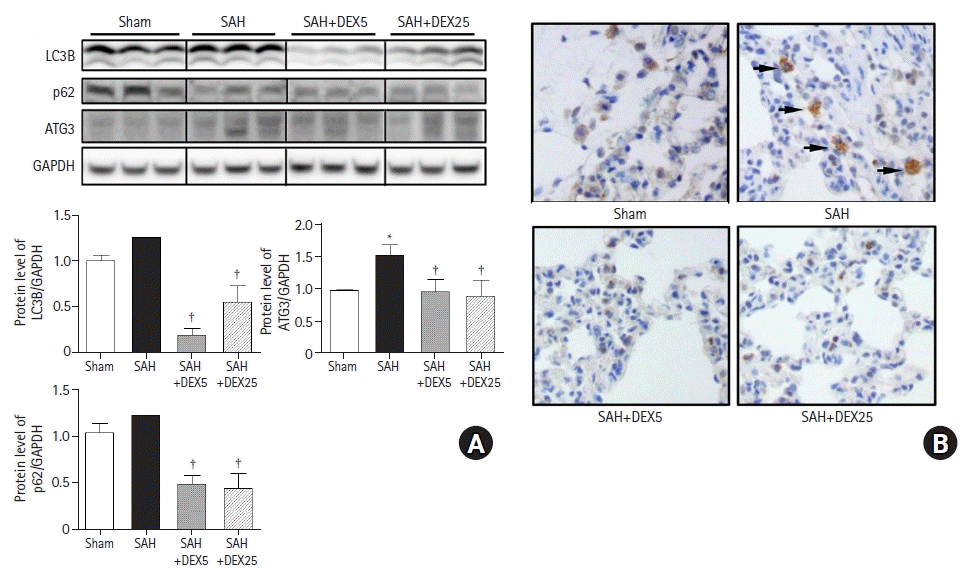
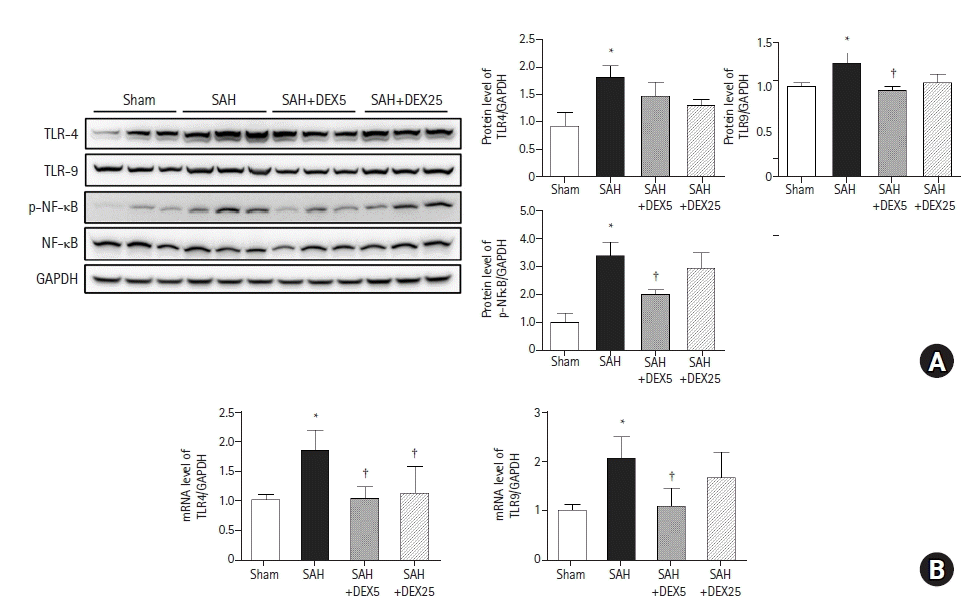
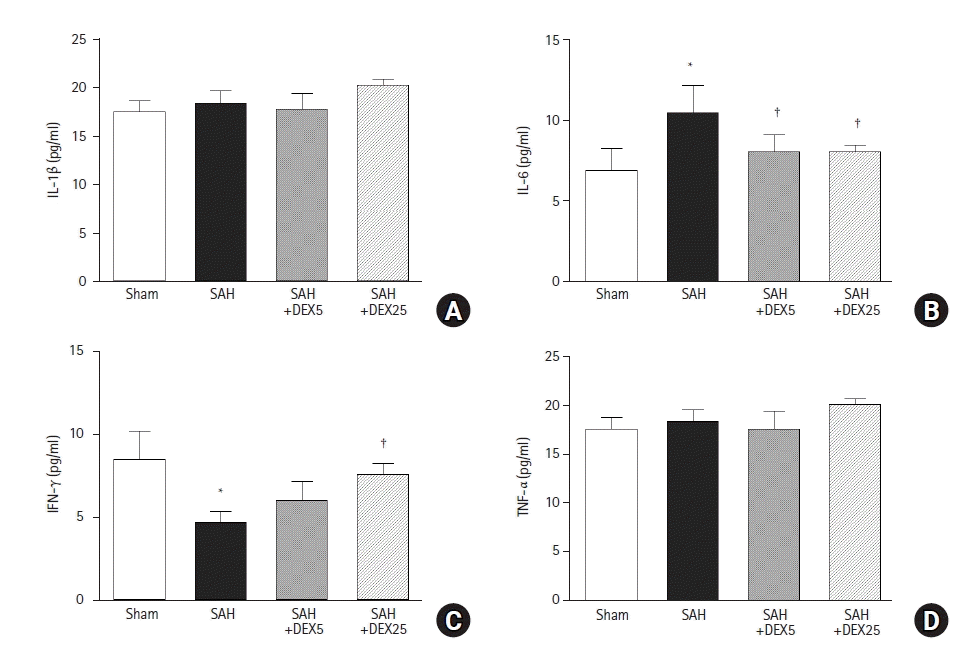




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download