This article has been
cited by other articles in ScienceCentral.
Abstract
Background
Sinking skin flap syndrome (SSFS) is a rare complication of decompressive craniectomy (DC) and causes a wide range of neurological deficits. Its pathophysiology remains debatable, however cranioplasty may decrease the symptoms of SSFS by reducing the direct effect of atmospheric pressure on the brain and allowing the correction of cerebral blood flow (CBF) and cerebral metabolism.
Case Report
A 36-year-old female patient underwent DC for a right frontal hematoma and signs of increased intracranial pressure following the resection of a right frontal arteriovenous malformation. Subsequently, she developed SSFS, altered neurological status with a Glasgow Coma Scale (GCS) score of 8/15, and bacterial-resistant meningitis. We applied a custom-shaped negative pressure helmet on the bone defect and used invasive and noninvasive techniques to measure the changes in intracranial pressure, CBF and cerebral oxygen saturation. Despite improvements in the cerebral physiological parameters, the neurological status did not improve (GCS score, 8/15).
Conclusion
To our knowledge, this is the first reported case of SSFS treated with a negative-pressure helmet and subsequent changes in cerebral parameters that were monitored invasively and noninvasively.
Go to :

Keywords: Cerebral blood flow, Critical care, Near-infrared spectroscopy, Decompressive craniectomy
INTRODUCTION
Sinking skin flap syndrome (SSFS) corresponds to altered neurological status following decompressive craniectomy (DC), which results in a persistent bone defect. It was first described in 1939 by Grant and Norcross as syndrome of the trephined. Since 1977 and following the work of Yamaura and Makino [
1], the term “SSFS” has been commonly used to describe this entity. Patients with SSFS may experience a wide range of neurological symptoms, which may vary from motor impairments, cognitive disturbances, impaired vigilance, headache and sensory impairments to visual symptoms or seizures [
2,
3]. Clinically or radiologically, physicians can observe concaving or sinking of the scalp to a plane that is lower than the edges of the skull defect [
3,
4].
SSFS may appear 3–10 months after DC or, in rare cases, a few days or weeks after a lumbar puncture, ventricular derivation or removal of a bone flap [
2,
5-
7]. The pathophysiology of SSFS remains unclear and debatable, even after the works of Yoshida et al. [
8] in 1996 and Winkler et al. [
9] in 2000, respectively. The fact that, in most cases, correction of the bone defect by cranioplasty results in improvements or complete recovery is in favor of a direct effect of atmospheric pressure on cerebral blood flow (CBF) and cerebral metabolism [
2,
3,
6,
7].
Herein, we describe a case of SSFS following surgical resection of an arteriovenous malformation (AVM) complicated by increase in intracranial pressure (ICP) and DC due to postoperative cerebral hematoma. In the weeks following the intervention, the patient developed persistently altered neurological status (somnolence without any response and Glasgow Coma Scale (GCS) decreased form 7-9/15 to 4/15), which correlated with the findings of cerebral tomodensitometry (TDM), such as right-sided large bone defect with sub-falcorial amygdala and temporal engagement (
Fig. 1). SSFS was diagnosed but cranioplasty was contraindicated because of bacterial meningitis. To improve the neurological status, we used a negative-pressure helmet and monitored the effects on the clinic, ICP, brain tissue oxygen tension, and CBF.
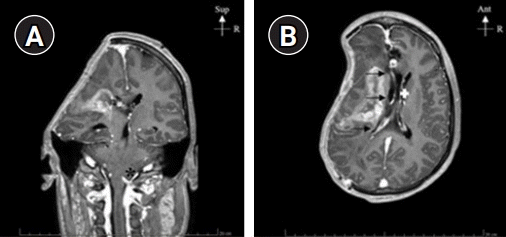 | Fig. 1.Magnetic resonance imaging T1-weighted images showing the bone defect area of the skull of the patient. (A) Coronal T1-weighted view shows a left-sided deviation of the midline and a filling of the posterior fossa with amygdala herniation (asterisk). (B) Axial T1 view shows left-sided deviation (arrows) and shrinking of lateral ventricles (white cross). Sup, superior; R, right; Ant, anterior. 
|
Go to :

CASE REPORT
A 36-year-old female patient with well-controlled high blood pressure and history of pneumothorax pleurodesis presented in early 2021 with an index seizure caused by an unknown AVM of 3.5-cm diameter in the axial plane located in the right frontal sulcus. The AVM was graded as a stage 2 according to the Spetzler-Martin classification. It was fed by the right anterior cerebral, right Sylvian, and dural (anterior meningeal, middle meningeal, and superficial temporal) arteries, and drained by three superficial frontal veins. Antiepileptic medication was introduced with no recurrence of seizures. Before surgical resection of the AVM, the patient underwent four sessions of neuroradiological embolization to limit its blood supply.
In the immediate postoperative period after the surgical resection of the AVM, no complications or neurological deficits were observed. Three days later, the GCS decreased to 8/15, and cerebral TDM was performed. The presence of a voluminous cerebral right frontal hematoma with simultaneous vasogenic cerebral edema and elevated ICP was observed, which explained the neurological impairments. Consequently, DC was performed, which was complicated by a massive perioperative hemorrhage. An external ventricular drain (EVD) was placed due to the presence of hemoventricle.
The postoperative period was further complicated by persistently raised ICP due to cerebral edema and intraventricular hemorrhage, which necessitated the placement of two new EVDs. Two weeks later, the patient presented with diffuse beta-lactamase-producing Escherichia coli meningitis. Meropenem and gentamycin were administered for 4 weeks followed by a combination of ceftazidime and avibactam (Zavicefta; Pfizer, New-York, NY, USA) for 3 weeks.
Two months after the resection of the AVM, the altered neurological status persisted (GCS 8-10/15) without any signs of improvement. Magnetic resonance imaging revealed the presence of a sub-falcorial amygdala and temporal engagement that were indicative of SSFS. Therefore, we attempted to reshape the skull to improve the neurological status.
Before ordering the custom-shaped helmet, we obtained a signed agreement from her legal representative in accordance with our local ethical committee. At the time of assessments, our patient was in the neuroscience intensive care unit, intubated, and non-sedated. Monitoring included oxygen monitoring, 5-lead electrocardiogram, radial invasive blood pressure and EVD that continuously measured the ICP.
We used the Luciole monitoring solution (Luciole Medical, Zurich, Switzerland) to monitor the changes in cerebral parameters. This solution includes a frontal skin patch (RheoPatch, Luciole Medical) that uses near-infrared spectroscopy (NIRS) to noninvasively estimate cerebral oxygen saturation (SbtO2) and CBF. Luciole monitoring allows the use of intravenously injected indocyanine green (ICG) dye dilution to improve the accuracy CBF. Therefore, we injected ICG (15 mg) before and after the application of negative pressure to determine the changes in CBF.
During the measurements, the patient was lying supine with head tilted up at 30°. We gently applied the Lenoir Orthopédie (Daniel Robert Orthopédie, Geneva, Switzerland) negative-pressure helmet. The helmet is shaped according to the size of the bone defect and comprises of three components (
Fig. 2). The first component is a silicon layer that softly attached to the edges of the bone defect, the second component is the vacuum, and the last component is a plastic layer that is shaped in the form of the normal shape of the patient skull. A manual vacuum pump (Aircast walker like pump) was connected to act like a balloon. While pumping, progressive increase in negative pressure allows for re-expansion of the dura mater and reshaping of the skull.
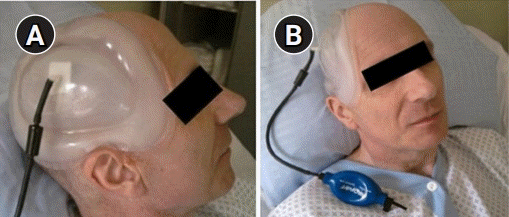 | Fig. 2.The Lenoir Orthopédie negative-pressure helmet with a manual vacuum pump. Images obtained from Lenoir Orthopédie user manual (Lenoir Orthopédie, Daniel Robert Orthopédie, Geneva, Switzerland). 
|
Unfortunately, even after the application of the negative pressure and improvements of cerebral parameters, no clinical improvement was observed. The neurological clinical status remained unchanged (GCS score, 8/15), and no reaction to painful stimulation was observed. The long-standing increase in ICP and decrease in CBF resulted in profound cerebral and ischemic lesions that could not be reversed by our mechanical solution. Due to unfavorable outcomes and in accordance with the wishes of the patient’s family, supportive therapies were discontinued.
After applying the helmet on the edges of the skull defect, we progressively decreased the pressure in the balloon according to the hemodynamic tolerance of the patient and registered the clinical parameters for 6 minutes. The heart rate, oxygen saturation (using a pulse oximeter), and mean arterial pressure did not change significantly during the procedure. The ICP significantly decreased from 6 cmH
2O to –3 cmH
2O with a simultaneous increase in cerebral perfusion pressure (CPP) from 62 mmHg to 73 mmHg. Indirect SbtO
2 and CBF parameters increased to normal values (
Fig. 3). CBF increased from 50 mL/g to 65 mL/g and the cerebral saturation on the left side of the forehead of the patient increased by eight points (from 64% to 72%) (
Fig. 4).
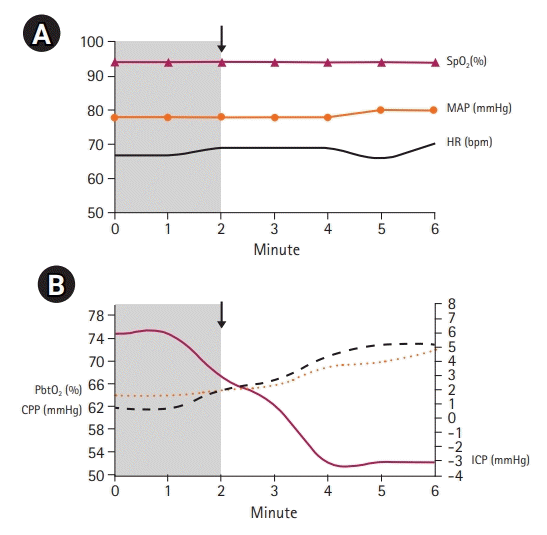 | Fig. 3.Hemodynamic and cerebral monitoring before and after the application of negative pressure using a Lenoir Orthopédie helmet. Grey zone, the time periods before the application of the negative pressure; arrow, start application of negative pressure. The procedure was hemodynamically supported and revealed reduction of intracranial pressure (ICP) to normal values and improvements in cerebral perfusion pressure (CPP) and cerebral oxygen saturation. (A) Hemodynamic parameters before and after applying negative pressure. (B) Cerebral hemodynamic parameters before and after applying negative pressure. SpO2, pulse oximeter; MAP, mean arterial pressure; HR, heart rate; PbtO2, brain tissue oxygen tension. 
|
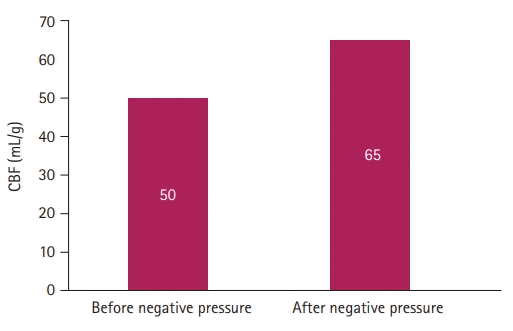 | Fig. 4.Increase in cerebral blood flow (CBF) after applying negative pressure. The CBF increased from 50 mL/g to 65 mL/g. 
|
Go to :

DISCUSSION
SSFS is a rare complication of DC. However, its pathophysiological mechanism remains unclear. Some authors believe that this may be caused by the direct transmission of atmospheric pressure to the intracranial cavity, which results in a decrease in CBF in the area of bone defect. Decreased blood flow and the consequent impaired cerebral metabolism may explain the symptoms of SSFS. According to other authors, SSFS is a consequence of a pressure decrease in the cerebral spinal fluid (CSF) compartment, which is worsened by orthostatic changes in position [
2,
3,
5,
6]. In this case, SSFS resulted in the stretching of fiber bundles and cranial nerves owing to the direct effects of atmospheric pressure on the brain. The subsequent symptoms occur when the patient changes position from a horizontal to an upright position [
10]. Cranioplasty may restore CBF by reshaping the skull and protecting from atmospheric pressure. Furthermore, improvements in CSF flow and cerebral metabolism have been elegantly demonstrated using TDM scans, TDM perfusion imaging and xenon TDM [
2,
3]. Improvements in CSF velocity have been reported after the closure of the bone defect. Dujovny et al. explained this modification to be a consequence of changes in the compliance of the craniospinal system [
6].
In this report, we first described the correction of SSFS by applying negative pressure and measuring the direct physiological changes in CBF, ICP, and SbtO2 using NIRS monitoring. Our patient could not undergo cranioplasty due to bacterial meningitis, and we used a negative-pressure helmet to treat SSFS. This system permits the reshaping of the cranial flap by creating negative pressure in the area of the bone defect. It can be used in cases of complete skull defects or the presence of a cranial flap. In the latter, the helmet must be custom-made.
In our case, when the skull was reshaped using the negative-pressure helmet, the ICP changed from a positive pressure to negative pressure of –3 cmH2O. This negative pressure restored the conditions of a normally closed skull and led to decompression of the brain, which was previously subjected to direct atmospheric pressure.
The parameters of CPP and SbtO
2 also improved. As expected, reshaping of the skull was associated with a reduction in the ICP and subsequent improvements in CPP, CBF, and SbtO
2. These results correlate with the assumption that cranioplasty restores cerebral perfusion and CSF dynamics. Similar results were observed by Yoshida et al. [
8] and Winkler et al. [
9] who used
33Xe computed tomography and
31P magnetic resonance spectroscopy as well as transcranial Doppler (TCD) and positron emission tomography to assess changes in physiological parameters [
8,
9]. In our report, we used another bedside noninvasive method to monitor CBF using intravenous ICG and Fick’s principle. This novel monitoring is presently being evaluated for this indication, but it can be a promising technique [
11-
13].
Nowadays, assessment of CBF using TCD is being performed when facing rapid neurological changes following extra-dural or subarachnoid hematomas or even after cerebral aneurysm rupture [
14,
15]. Nevertheless, studies have demonstrated a good correlation between changes in CBF using NIRS and those measured by TCD.
Cranioplasty is indicated in cases of symptomatic SSFS following DC. When facing SSFS complicated with bacterial meningitis, the use of a negative-pressure helmet appears to restore cerebral physiologic parameters and may be a helpful intervention. Using this approach, invasive and noninvasive neuromonitoring (ICP and NIRS with ICG) confirmed changes in cerebral metabolism and blood flow. Unfortunately, we observed no clinical improvement because of the profound and irreversible cerebral lesions.
Go to :







 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download