1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.

3. Park CH, Yang DH, Kim JW, et al. Clinical practice guideline for endoscopic resection of early gastrointestinal cancer. Clin Endosc. 2020; 53:142–166.

4. Ko BM. History and development of accessories for endoscopic submucosal dissection. Clin Endosc. 2017; 50:219–223.

5. Song JH, Kim SG. Endoscopic submucosal dissection for gastric neoplasia. In : Chun HJ, Yang SK, Choi MG, editors. Therapeutic gastrointestinal endoscopy: a comprehensive atlas. Singapore: Springer;2019. p. 125–141.
6. Sumiyama K, Tajiri H. History of ESD. In : Fukami N, editor. Endoscopic submucosal dissection: principles and practice. New York: Springer;2015. p. 3–8.
7. Gotoda T, Iwasaki M, Kusano C, et al. Endoscopic resection of early gastric cancer treated by guideline and expanded National Cancer Centre criteria. Br J Surg. 2010; 97:868–871.

8. Kamiya S, Rouvelas I, Lindblad M, et al. Current trends in gastric cancer treatment in Europe. J Cancer Metastasis Treat. 2018; 4:35.

9. Draganov PV, Wang AY, Othman MO, et al. AGA Institute Clinical Practice Update: endoscopic submucosal dissection in the United States. Clin Gastroenterol Hepatol. 2019; 17:16–25.

10. Fukami N. Endoscopic submucosal dissection: principles and practice. New York: Springer;2015.
11. Oda I, Suzuki H, Nonaka S, et al. Complications of gastric endoscopic submucosal dissection. Dig Endosc. 2013; 25(Suppl 1):71–78.

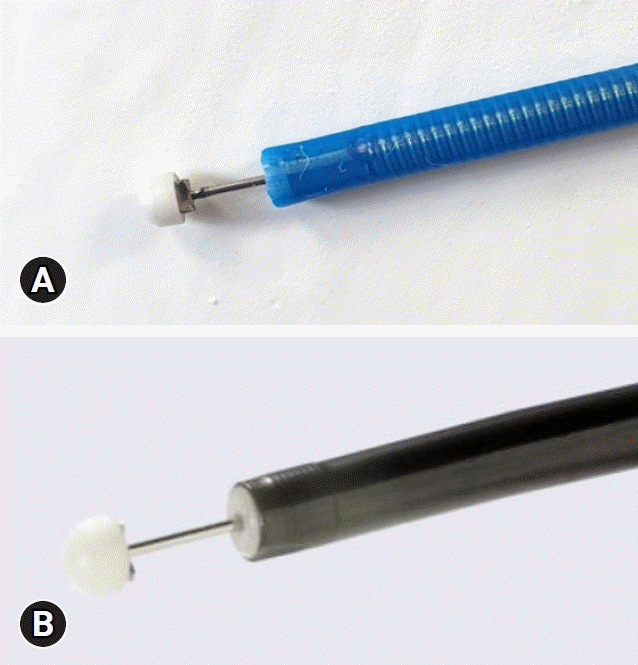
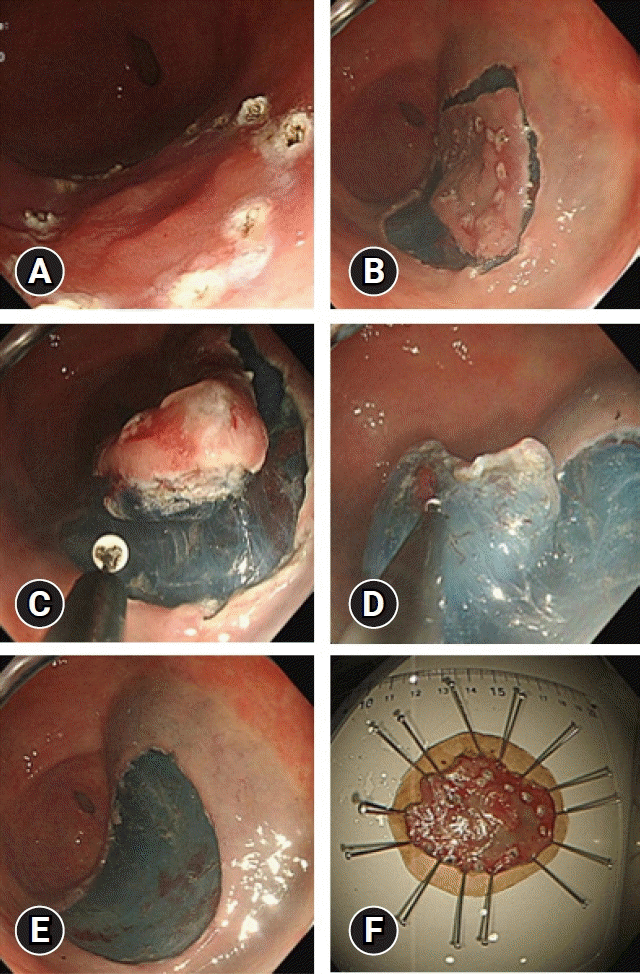
12. Choi HS, Chun HJ. Accessory devices frequently used for endoscopic submucosal dissection. Clin Endosc. 2017; 50:224–233.

13. Fukami N. Appendix: commonly used ESD knives. In : Fukami N, editor. Endoscopic submucosal dissection: principles and practice. New York: Springer;2015. p. 257–260.
14. Hosokawa K, Yoshida S. Recent advances in endoscopic mucosal resection for early gastric cancer. Gan To Kagaku Ryoho. 1998; 25:476–483.
15. Cho JY, Cho WY. The current status of endoscopic submucosal dissection. Korean J Gastrointest Endosc. 2008; 37:317–320.
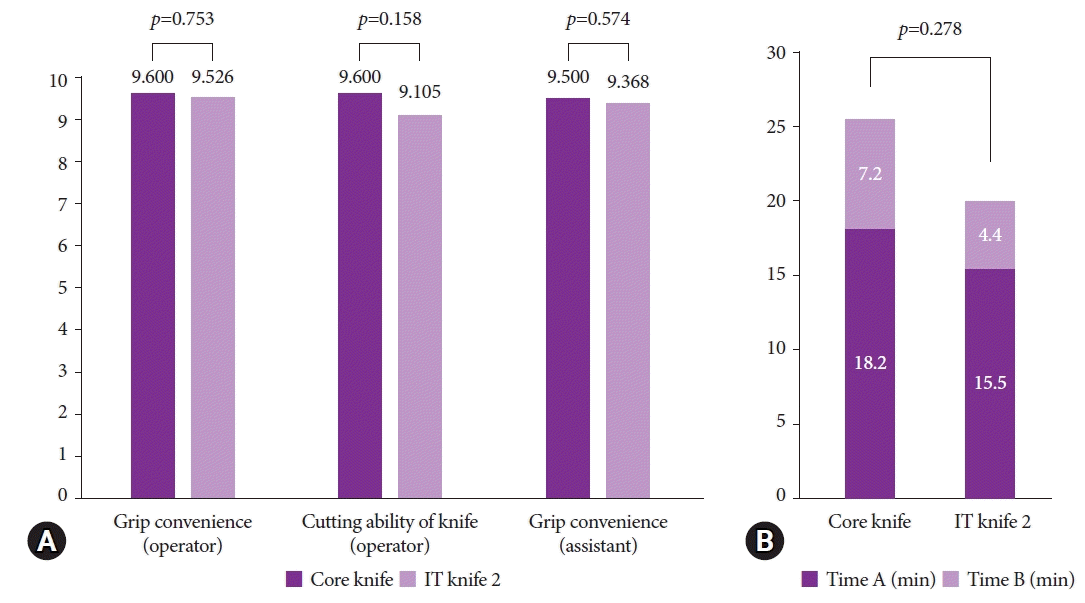
16. Konuma H, Matsumoto K, Ueyama H, et al. Procedure time for gastric endoscopic submucosal dissection according to location, considering both mucosal circumferential incision and submucosal dissection. Gastroenterol Res Pract. 2016; 2016:9183793.

17. Kim KO, Kim SJ, Kim TH, et al. Do you have what it takes for challenging endoscopic submucosal dissection cases? World J Gastroenterol. 2011; 17:3580–3584.

18. Lee SG, Cho KB, Hong YS, et al. Nonsurgical treatment of gastric perforation complicated by endoscopic mucosal resection and endoscopic submucosal dissection. Korean J Gastrointest Endosc. 2008; 37:97–104.
19. Park YS, Park SW, Song SY, et al. Endoscopic mucosal resection using insulated-tip electrosurgical knife. Korean J Gastrointest Endosc. 2003; 26:397–404.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download