INTRODUCTION
Pancreatic cystic lesions (PCLs) are increasingly being diagnosed owing to the widespread use of imaging tests for evaluating gastrointestinal symptoms or for other purposes. PCLs are incidentally diagnosed in up to 20% of patients undergoing abdominal imaging for other etiologies.
1-
4 Increased awareness among physicians and improved quality of cross-sectional imaging modalities, such as computed tomography and magnetic resonance imaging, also contribute to the increased prevalence of PCLs.
5,
6 The three major subtypes of PCLs are pseudocysts, non-mucinous cystic lesions, and mucinous cystic lesions. Mucinous cysts mainly include intrapapillary mucinous cystic neoplasms and mucinous cystic neoplasms, which have a higher potential for malignant transformation. Solid pseudopapillary neoplasms and cystic neuroendocrine tumors are also PCLs; however, they usually have some solid components and do not typically present as classic cystic lesions. Endoscopic ultrasound (EUS) not only provides additional higher-definition information on the morphological features of the cyst but can also be used for fine-needle aspiration (FNA) to obtain cyst fluid for biochemical, cytological, and molecular analyses.
6,
7
Early data showed significant cyst infection rates of up to 14% after EUS-guided FNA (EUS-FNA) of PCLs.
8 On the basis of the initial studies, major gastroenterological societies recommended the prophylactic use of periprocedural and postprocedural antibiotics in patients undergoing EUS-FNA of pancreatic cysts. The European Society of Gastrointestinal Endoscopy and American Society for Gastrointestinal Endoscopy guidelines on antibiotic prophylaxis after EUS-FNA of PCLs provided weak recommendations based on low-quality evidence.
9,
10 Subsequent studies showed that previously reported infection rates were overestimated and questioned the benefit of antibiotic prophylaxis after EUS-FNA of PCLs.
11,
12
The primary aim of this study was to compare the infection rate between patients who received postprocedural prophylactic antibiotics and those who did not, regardless of periprocedural antibiotic administration, and to assess the benefit of postprocedural antibiotic prophylaxis.
Go to :

METHODS
Study design
Prospectively collected data of patients who underwent EUS-FNA of pancreatic cysts between January 2015 and July 2019 were retrospectively extracted from the patient databases of two large academic centers (Memorial Hermann Hospital at Texas Medical Center, Houston, TX, USA; Beaumont Health, Royal Oak, MI, USA). Both institutions are state-of-the-art tertiary-care academic centers. All procedures were performed by experienced endosonographers (NT, SR, and MEC). The standard of care at both hospitals is complete drainage of the cyst until it completely collapses, as observed on the EUS monitor. Linear echoendoscopes (GF-UCT 160 and GF-UCT 180; Olympus America, Center Valley, PA, USA) and an Aloka processor were used at William Beaumont Hospital, and Pentax linear EUS (EG-3870UTK; Pentax Medical, Montvale, NJ, USA) scopes and a Hitachi processor were used at Memorial Hermann. Data were collected by reviewing the patients’ electronic medical records, EUS procedure reports, postprocedural recommendations, pre- and postprocedural anesthesia reports, follow-up notes, pertinent imaging findings, and telephone encounters. Procedure-specific details, including gauge of the needle used, number of needle passes into the cyst, and number of drained cysts, were also collected. The rate of systemic or local infection was calculated by checking all records in the Memorial Hermann and Beaumont Health systems for any patient note, telephone encounter, communication/correspondence note, or external media records mentioning cyst infection or systemic infection occurring in the first 60 days after the procedure, even if the note was dated >60 days from the date of the procedure. In addition, abdominal abscesses detected on cross-sectional imaging, positive blood cultures, and febrile episodes occurring within 60 days after the procedure were evaluated. Serum lipase levels, imaging data, and patient charts were also checked for other complications, such as perforation, pancreatitis, peritonitis, or abdominal pain. Two reviewers (SH and MG) reviewed the extracted data and patient charts, and a third reviewer (SR) independently reviewed the extracted data. Adults (age ≥18 years) with PCLs who underwent EUS-FNA were considered eligible for inclusion. Patients with age <18 years, known active infection or sepsis before the endosonographic examination, infected pseudocysts, pancreatic cysts that were not subjected to needle aspiration, pancreatic solid lesions, or mixed solid–cystic pancreatic lesions were excluded.
Study groups
All adults who underwent inpatient or outpatient EUS-FNA of PCLs were included in the study. One arm of the study included patients who received antibiotics for prophylaxis for 3 to 5 days after the EUS-FNA examination at our health-care systems (POSTAB+ group). The other arm included patients who did not receive antibiotics after the EUS-FNA examination regardless of periprocedural antibiotic administration at both health-care systems (POSTAB– group). At both hospitals, most of the patients in the two study arms received one dose of periprocedural antibiotics for prophylaxis.
Antibiotic administration
Each center has its own protocol for antibiotic prophylaxis during and after EUS-FNA of PCLs. In particular, most of the patients at Beaumont Health received prophylactic antibiotics for 3 to 5 days (mainly oral fluoroquinolones) after the procedure, contrary to patients at Memorial Hermann who did not routinely receive prophylactic antibiotics after the procedure. Most patients at both centers received one dose of periprocedural antibiotics (mainly intravenous [IV] fluoroquinolones).
Outcomes
The primary outcome of this study was the rate of cyst infection after EUS-FNA of PCLs in the two groups. The secondary outcomes included the rate of systemic infection after EUS-FNA; efficacy of postprocedural antibiotic prophylaxis in reducing both local and systemic infections after EUS-FNA of pancreatic cysts; and other procedure-related adverse events such as perforation, pancreatitis, peritonitis, or abdominal pain. Infectious complications were either local (cyst infection) or systemic (bacteremia or sepsis). Cyst infection was defined as the presence of evidence of abscess on imaging, EUS, or fluid analysis or culture. Systemic infection was defined as positive blood cultures after the procedure or new-onset persistent fever after the procedure with no evidence of local infection, and after ruling out pancreatitis if the patients did not meet two of the three Atlanta criteria for diagnosing acute pancreatitis.
Statistical analyses
The results are reported as mean±standard deviation or median with interquartile range for continuous data, depending on whether the data distribution was parametric or non-parametric. For categorical variables, the results are expressed as counts and frequencies (%). Most of the data were based on the comparison between patients who received postprocedural antibiotics for prophylaxis and those who did not. Statistical analysis was performed using the Stata statistical software (ver. 14.2; StataCorp. LP, College Station, TX, USA).
Ethical statements
The study protocol was approved by the institutional review board at both institutions (William Beaumont Hospital, Royal Oak, Michigan; Memorial Hermann Hospital at Texas Medical Center, Houston, Texas).
Go to :

RESULTS
The initial search of the databases at both institutions yielded 3,593 EUS procedures performed during the study period. After applying the inclusion and exclusion criteria, we determined that 470 of the 3,593 procedures were EUS-FNA performed in patients with pancreatic cysts (
Fig. 1). Twenty-two patients underwent a repeat EUS-FNA examination of pancreatic cysts within the study period; hence, 448 patients underwent 470 procedures. Of the 448 patients, 263 were women (58.7%). The mean age of the study population was 66.3±12.8 years. Most of the patients had one cyst, with a mean cyst size of 25.7±16.9 mm. The most common location of the cyst was the head of the pancreas, followed by the body and tail. The most common needle size used was 22-gauge needle. The mean number of needle passes for EUS-FNA of pancreatic cysts was 1.26±0.66 (
Table 1).
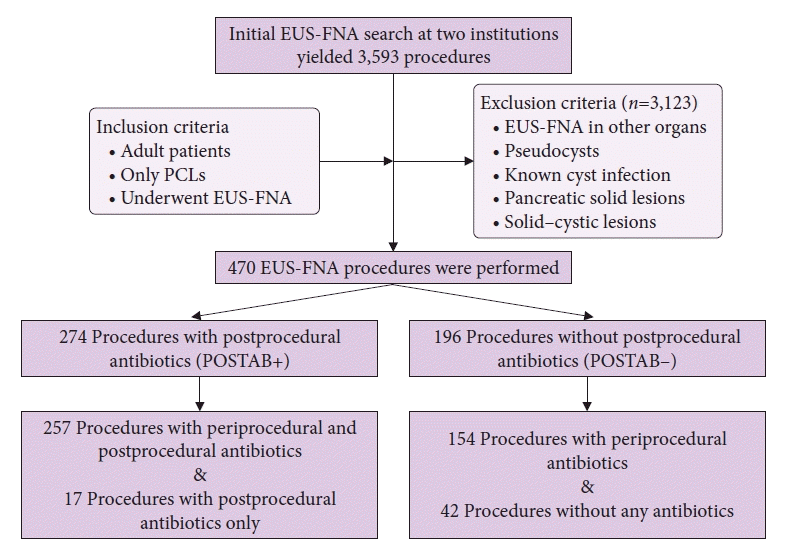 | Fig. 1.Study flow diagram. EUS-FNA, endoscopic ultrasound–guided fine-needle aspiration; PCLs, pancreatic cystic lesions; POSTAB+, postprocedural antibiotic group; POSTAB–, no postprocedural antibiotic group. 
|
Table 1.
Patients, pancreatic cysts, and procedure characteristics
|
Characteristic |
Total |
POSTAB+ |
POSTAB– |
p-value |
|
No. of patients |
448 (100.0) |
273 (60.9) |
175 (39.1) |
|
|
Women |
263 (58.7) |
155 (58.9) |
108 (41.1) |
|
|
No. of procedures |
470 (100.0) |
274 (58.3) |
196 (41.7) |
|
|
Mean age (yr) |
66.3±12.8 |
66.4±12.5 |
66.4±13.3 |
|
|
Mean cyst size (mm) |
25.7±16.9 |
26.1±18.6 |
25.2±14.3 |
0.302 |
|
≤20 |
200 (42.6) |
114 (41.6) |
86 (43.9) |
|
20–30 |
141 (30.0) |
81 (29.6) |
60 (30.6) |
|
30–40 |
63 (13.4) |
37 (13.5) |
26 (13.3) |
|
40–50 |
58 (12.3) |
40 (14.6) |
18 (9.2) |
|
≥50 |
8 (1.7) |
2 (0.7) |
6 (3.1) |
|
No. of cysts |
|
|
|
0.316 |
|
One cyst |
339 (72.1) |
193 (70.4) |
146 (74.5) |
|
Two cysts |
67 (14.3) |
38 (13.9) |
29 (14.8) |
|
More than 2 cysts |
64 (13.6) |
43 (15.7) |
21 (10.7) |
|
Cyst location |
|
|
|
0.140 |
|
Head |
143 (30.4) |
83 (30.3) |
60 (30.6) |
|
Uncinate |
40 (8.5) |
19 (6.9) |
21 (10.7) |
|
Neck |
66 (14.0) |
36 (13.1) |
30 (15.3) |
|
Body |
134 (28.5) |
75 (27.4) |
59 (30.1) |
|
Tail |
87 (18.5) |
61 (22.3) |
26 (13.3) |
|
Mean needle passes |
1.3±0.7 |
1.3±0.7 |
1.2±0.5 |
|
|
Needle size |
|
|
|
0.01 |
|
19-gauge needle |
34 (7.2) |
15 (5.5) |
19 (9.7) |
|
22-gauge needle |
406 (86.4) |
246 (89.8) |
160 (81.6) |
|
25-gauge needle |
30 (6.4) |
13 (4.7) |
17 (8.7) |

A total of 411 patients (87.4%) received one dose of periprocedural IV antibiotics, and 59 patients (12.5%) did not receive periprocedural antibiotics. Of these 59 patients, 42 patients also did not receive postprocedural antibiotics for prophylaxis. Thus, 42 of the 470 patients (8.9%) underwent EUS-FNA of PCLs without any antibiotic administration.
A total of 274 patients (58.3%) received antibiotics for 3 to 5 days after EUS-FNA of PCLs, and they were identified as the POSTAB+ group. Meanwhile, 196 patients (41.7%) did not receive antibiotics after EUS-FNA of PCLs, and they were identified as the POSTAB– group (
Table 1). In the periprocedural period, the most commonly used antibiotics were IV fluoroquinolones (88.6%), followed by IV cephalosporins (7.3%). If prophylactic antibiotics were used after the procedure, oral fluoroquinolones (93.8%) followed by oral cephalosporins (2.9%) were the most commonly administered antibiotics for 3 to 5 days.
None of the patients in either group (POSTAB+ or POSTAB–) developed any localized pancreatic cyst infection. In addition, none of the patients reported symptoms or signs suggestive of systemic infection. Furthermore, none of the 42 patients who did not receive any dose of periprocedural or postprocedural antibiotics developed localized or systemic infection.
As shown in
Table 2, a total of 11 patients (2.3%) experienced adverse events after the EUS-FNA procedure; however, none of the events were related to the antibiotic administration status. One of the 11 patients had a serious adverse event: postprocedural bowel perforation complicated by peritonitis that required prolonged and complicated hospital admission and surgical intervention. The remaining 10 patients had other mild adverse events, as follows: intra-abdominal hematoma in one patient, which spontaneously resolved; mild pancreatitis in one patient, which required hospital admission and was conservatively managed, resulting in the patient’s discharge within 48 hours; and mild abdominal pain that did not require hospital admission in the remaining 8 patients. Pancreatitis was ruled out in these eight patients based on serum lipase measurement and contrast-enhanced abdominal cross-sectional imaging findings. None of the patients in this study had any procedure-related infections. Two patients developed adverse events; however, these were not attributed to the procedure itself or to the antibiotic administration status. One of the two patients died 3 days after EUS-FNA from causes related to underlying comorbidities. The other patient developed pancreatitis; however, this patient underwent endoscopic retrograde cholangiopancreatography on the same day as EUS-FNA. Therefore, the pancreatitis was likely post-endoscopic retrograde cholangiopancreatography pancreatitis (
Table 2). In a sub-analysis of the 42 patients who did not receive any antibiotics, only two patients reported abdominal pain, which spontaneously resolved.
Table 2.
Outcomes of post-procedural antibiotic group versus no post-procedural antibiotics group complications
|
Variable |
Total |
POSTAB+ |
POSTAB– |
p-value |
|
No. of procedures |
470 (100.0) |
274 (58.3) |
196 (41.7) |
|
|
Periprocedural antibiotics |
|
|
|
<0.01 |
|
Yes |
411 (87.4) |
257 (93.8) |
154 (78.6) |
|
|
No |
59 (12.6) |
17 (6.2) |
42 (21.4) |
|
|
Fluoroquinolones |
364 (88.6) |
232 (90.3) |
132 (85.7) |
|
|
Cephalosporins |
30 (7.3) |
17 (6.6) |
13 (8.4) |
|
|
Clindamycin |
9 (2.2) |
6 (2.3) |
3 (1.9) |
|
|
Other |
8 (1.9) |
2 (0.8) |
6 (3.9) |
|
|
Postprocedural antibiotics |
274 (100.0) |
274 (100.0) |
|
NA |
|
Fluoroquinolones |
257 (93.8) |
257 (93.8) |
|
|
|
Cephalosporins |
6 (2.2) |
6 (2.2) |
|
|
|
Clindamycin |
4 (1.5) |
4 (1.5) |
|
|
|
Others |
7 (2.6) |
7 (2.6) |
|
|
|
Postprocedural complications |
11 (2.3) |
4 (1.5) |
7 (3.6) |
NA |
|
Infections |
0 |
0 |
0 |
|
|
Pancreatitis |
1 (0.2) |
0 |
1 (0.5) |
|
|
Intra-abdominal hematoma |
1 (0.2) |
0 |
1 (0.5) |
|
|
Abdominal pain |
8 (1.7) |
3 (1.1) |
5 (2.6) |
|
|
Bowel perforation and peritonitis |
1 (0.2) |
1 (0.4) |
0 |
|
|
Complications requiring hospital admission |
2 (0.4) |
1 (0.4) |
1 (0.5) |
NA |
|
Complications not related to the procedure or antibiotic status |
2 (0.4) |
1 (0.4) |
1 (0.5) |
NA |
|
Death after 3 days from causes not related to the procedure |
1 (0.2) |
1 (0.4) |
0 |
|
|
Pancreatitis after ERCP |
1 (0.2) |
0 |
1 (0.5) |
|

Go to :

DISCUSSION
Accurate diagnosis of PCL subtypes is crucial for further management because certain PCLs tend to have a higher malignancy risk. Some cystic lesions require close surveillance or surgical resection. Many guidelines have been published to help guide the management of pancreatic cysts, which mainly include, but are not limited to, the International Association of Pancreatology Sendai guidelines in 2006,
13 Fukuoka guidelines in 2012,
14 American Gastroenterological Association guidelines in 2015,
15 revised Fukuoka guidelines in 2017,
16 American College of Gastroenterology guidelines in 2018,
17 and the European Society of Gastrointestinal Endoscopy guidelines in 2018.
4 FNA plays a pivotal role in the accurate diagnosis and management of PCLs.
The cyst infection rate after EUS-FNA of PCLs is inconsistently reported. The initially reported infection rate was overestimated (14%),
8 and subsequent studies showed that the incidence of systemic or pancreatic cyst infection was significantly lower than that in the initial report. The negligible incidence of systemic infection after EUS-FNA in gastrointestinal organs or other organs near the gastrointestinal tract has been substantiated by Barawi et al.,
18 who showed in their prospective study that the procedure was not associated with bacteremia or systemic infectious complications. Controversy still exists about antibiotic prophylaxis for patients undergoing EUS-FNA of PCLs, and the benefit of antibiotic prophylaxis remains questionable. The current American Society for Gastrointestinal Endoscopy guidelines recommend administration of antibiotics for 3 to 5 days after EUS-FNA of PCLs.
10 Furthermore, the ESGE still upholds their previous recommendation of 3–5 days of oral antibiotics for prophylaxis after EUS-FNA of PCLs.
9 Our study showed that the incidence of localized or systemic infection after EUS-FNA was negligible with a single dose of periprocedural IV antibiotics, without the need for 3 to 5 days of postprocedural antibiotics.
In 2005, Lee et al.
19 demonstrated no difference in infection rates between patients who received and those who did not receive prophylactic antibiotics; they reported one probable infection in the prophylactic antibiotic group but did not mention whether the infection was systemic or local. In 2011, Guarner-Argente et al.,
11 in their retrospective cohort study, showed no difference in the incidence of infectious complications with or without antibiotic prophylaxis in 253 patients who underwent 266 EUS-FNA procedures for PCLs; they reported only one cyst infection in the antibiotic arm and no cyst infection in the no-antibiotic arm. In an Australian case series of 85 EUS-FNA of PCLs published in 2014, Marinos et al.
20 reported no cyst infection with a single periprocedural IV antibiotic dose. Klein et al.,
21 in their study in 204 patients in 2017, demonstrated that a single IV dose of periprocedural antibiotic is safe and effective without the need for postprocedural prophylactic antibiotics; they reported only one case of infectious complication, in a patient who did not receive any prophylactic antibiotics. In 2019, Facciorusso et al.
12 demonstrated that prophylactic antibiotics did not reduce the risk of PCL infection after EUS-FNA examination in 270 propensity score-matched patients, and they recommended that routine prophylactic antibiotic use should be abandoned; they reported two cyst infections in the antibiotic arm and three cyst infections in the no-antibiotic arm. Finally, Colán-Hernández et al.,
22 in a multicenter, randomized, non-inferiority study in 205 patients, found that the incidence of infection did not significantly differ according to prophylactic antibiotic use; they reported zero cyst infection both in patients treated with ciprofloxacin and in those without ciprofloxacin prophylaxis after EUS-FNA of PCLs (
Table 3).
11,
12,
19-
22
Table 3.
|
Study |
Year of publication |
Study nature |
Study design |
Cyst infection |
AEs |
|
Lee et al.19
|
2005 |
Retrospective cohort study |
Prophylactic antibiotics were administered in 543 cases; no antibiotic was administered in 60 cases. |
1 Probable infection in the prophylactic antibiotic arm; no cyst infection in the no-antibiotic arm. |
13 AEs in the antibiotic group: pancreatitis (6), abdominal pain (4), infection (1), retroperitoneal bleed (1), and bradycardia (1). |
|
1 patient with pancreatitis underwent ERCP on the same day; 2 patients with abdominal pain underwent ERCP on the same day. |
|
Guarner-Argente et al.11
|
2011 |
Retrospective cohort study |
178 Procedures without any antibiotic prophylaxis versus 88 procedures with any antibiotic prophylaxis (periprocedural, postprocedural, or both). |
1 Cyst infection in the antibiotic arm; no cyst infection in the no-antibiotic arm. |
8 AEs in the antibiotic group: 2 severe AEs (1 cyst infection and 1 bile leak) and 6 mild AEs (2 local allergic reaction, 2 abdominal pain, 1 asymptomatic intracystic bleeding, and 1 Clostridium difficile diarrhea). |
|
12 AEs in the no-antibiotic group: 4 severe AEs (2 symptomatic local bleeding, 1 pancreatitis, and 1 bile leak) and 8 mild AEs (3 asymptomatic intracystic bleeding, 1 fever, 1 abdominal pain, 1 pharyngitis, and 2 sedation-related AEs). |
|
Marinos et al.20
|
2014 |
Prospective pilot study |
Single-arm study; 79 patients received a single dose of periprocedural IV prophylactic antibiotics. |
No cyst infection. |
8 AEs in the antibiotic group, all of which were mild: sedation-related AEs (2), neutrophilia requiring antibiotics (1), acute-on-chronic pancreatitis (1), abdominal pain (2), and sore throat (2). |
|
Klein et al.21
|
2017 |
Retrospective analysis |
146 Patients received single IV ceftriaxone (periprocedural); 23 patients received postprocedural antibiotics for 3 to 5 days; and 35 patients received no antibiotics. |
1 Infectious complication in the no-antibiotic arm (unknown if localized or systemic). |
6 AEs in the antibiotic arm, including pancreatitis (3) and abdominal pain (1). |
|
1 AE in the no-antibiotic arm (an infection; unknown if localized or systemic). |
|
2 patients received antibiotics for 3–5 days; 1 patient received IV ceftriaxone. |
|
Facciorusso et al.12
|
2019 |
Retrospective cohort study |
Propensity score-matched study. A total of 135 patients received both periprocedural IV antibiotics and postprocedural antibiotics for 3–5 days, whereas 135 patients did not receive any prophylactic antibiotics. |
2 Cyst infections in the antibiotic arm; |
10 AEs in the antibiotic group: 2 severe AEs (1 severe abdominal pain and 1 serious bleeding) and 8 non-serious AEs (2 cyst infection, 1 pancreatitis, 3 abdominal pain, and 2 antibiotic-related AEs). |
|
3 Cyst infections in the no-antibiotic arm. |
8 AEs in the no-antibiotic group: 1 serious cyst infection and 7 non-serious AEs (2 cyst infection, 3 abdominal pain, and 2 bleeding). |
|
Colán-Hernández et al.22
|
2020 |
Multicenter randomized non-inferiority trial |
103 Patients received both periprocedural and postprocedural prophylactic antibiotics, whereas 102 patients did not receive any prophylactic antibiotics. |
No cyst infection in the antibiotic arm; no cyst infection in the no-antibiotic arm. |
6 AEs in the antibiotic group: 1 moderate AE (pancreatitis) and 5 mild AEs (1 abdominal pain, 1 fever, 2 suspected tendinitis, and 1 diarrhea). |
|
8 AEs in the no-antibiotic group: 3 severe AEs (1 bacteremia, 1 accidental fall, and 1 pancreatitis); 1 moderate AE (bronchospasm); and 4 mild AEs (3 abdominal pain and 1 bleeding). |
|
Present study |
2022 |
Retrospective cohort study |
274 Cases with postprocedural prophylactic antibiotics and 196 cases without postprocedural prophylactic antibiotics. |
No cyst infection in both arms. |
4 AEs in the AB+ group: 3 mild AEs (all abdominal pain) and 1 severe AE (bowel perforation and peritonitis). |
|
7 AEs in the AB- group: all mild AEs (5 abdominal pain, 1 intra-abdominal hematoma, and 1 pancreatitis). |

The results of our study showed that the risk of infection after EUS-FNA of PCLs is very low, as demonstrated by the group of patients who did not receive postprocedural antibiotics for prophylaxis. Furthermore, this was supported by the lack of evidence of infection in a small subset of patients (n=42, 8.9%) who did not receive either periprocedural or postprocedural antibiotics. Thus, our data suggest that postprocedural prophylactic antibiotics have a limited role in patients undergoing EUS-FNA of pancreatic cysts.
The size and location of the PCLs were not associated with an increased risk of infection. In addition, the different needle sizes used for FNA and the route (transgastric or transduodenal) of FNA were not associated with an increased risk of infection. The complications reported in this study were related to the endoscopy procedure itself, rather than to the status of postprocedural antibiotic administration. The routine use of antibiotics may have some negative outcomes, such as added cost and, more important, increased drug resistance and increased risk of secondary infections (e.g., bacterial vaginosis or
Clostridium difficile infection). In addition, the use of antibiotics may increase the risk of allergic reactions, which can be life-threatening in certain situations.
20
The major strength of our study is that it is the largest study to date to evaluate the efficacy and safety of postprocedural antibiotic prophylaxis in patients undergoing EUS-FNA of pancreatic cysts. The results of this study support the findings of earlier studies performed by Guarner-Argente et al.,
11 Marinos et al.,
20 Klein et al.,
21 Facciorusso et al.,
12 and Colán-Hernández et al.
22 Therefore, this study can be considered a strong validation of previously published data. Lee et al.’s study was not designed to assess the efficacy of preprocedural or postprocedural antibiotics for prophylaxis, and the authors did not mention the status of antibiotic administration.
19 Guarner-Argente et al.’s study
11 focused more on the non-use of antibiotic prophylaxis, which is relatively different from our study. Although Marinos et al.’s study
20 was a prospective pilot study, it had a small cohort and was a single-arm study with no control arm. Meanwhile, Facciorusso et al.’s and Klein et al.’s studies
12,
21 are similar to our study; however, Facciorusso et al.
12 performed a propensity-matched score study, whereas our study had a larger sample size. Furthermore, the infection rate in the current study was similar to that in the recently published randomized multicenter, non-inferiority, prospective trial by Colán-Hernández et al.,
22 which showed that the incidence of infection was not affected by ciprofloxacin prophylaxis in patients who underwent EUS-FNA examination of PCLs. Compared with the study of Colán-Hernández et al.,
22 we believe that our study is well designed to assess the efficacy and safety of postprocedural antibiotic prophylaxis, and not the efficacy of periprocedural antibiotics, in reducing infectious complications. In addition, our study had a larger cohort than that of Colán-Hernández et al.,
22 although our study had a limitation of having a retrospective design.
The main limitation of our study was its retrospective nature. In addition, our study was not adequately powered to assess the safety of the non-use of antibiotic prophylaxis in patients undergoing EUS-FNA, despite the results of our sub-analysis in 42 patients. This study did not optimally assess adverse events or allergic reactions related to antibiotic administration owing to the retrospective analysis. Moreover, as most allergic reactions to antibiotics are mild and readily treated with antihistamines by anesthesiologists, the patients might not have recognized their symptoms as allergic reactions to medications. As a result, they might not have reported their symptoms to health-care providers or might have self-medicated with over-the-counter medications.
In conclusion, the risk of infection after EUS-FNA of pancreatic cysts is minimal, and prophylactic antibiotic administration after this procedure does not seem to offer additional benefits for preventing cystic or systemic infection. The safety of the non-use of antibiotic prophylaxis is beyond the scope of this study. Nevertheless, no infection was reported in the small subset of patients who did not receive any prophylactic antibiotics.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download