Abstract
Background/Aims
Patients with acute cholecystitis (AC) after metallic stent (MS) placement for malignant biliary obstruction (MBO) have a high surgical risk. We performed percutaneous transhepatic gallbladder aspiration (PTGBA) as the first treatment for AC. We aimed to identify the risk factors for AC after MS placement and the poor response factors of PTGBA.
Methods
We enrolled 401 patients who underwent MS placement for MBO between April 2011 and March 2020. The incidence of AC was 10.7%. Of these 43 patients, 37 underwent PTGBA as the first treatment. The patients’ responses to PTGBA were divided into good and poor response groups.
Results
There were 20 patients in good response group and 17 patients in poor response group. Risk factors for cholecystitis after MS placement included cystic duct obstruction (p<0.001) and covered MS (p<0.001). Cystic duct obstruction (p=0.003) and uncovered MS (p=0.011) demonstrated significantly poor responses to PTGBA. Cystic duct obstruction is a risk factor for cholecystitis and poor response factor for PTGBA, whereas covered MS is a risk factor for cholecystitis and an uncovered MS is a poor response factor of PTGBA for cholecystitis.
Cholecystitis occurs with a certain probability in patients treated with self-expandable metallic stent (SEMS) placement for distal malignant biliary obstruction (MBO).1,2 Cholecystitis is a well-known complication of stent insertion that reduces the patient’s quality of life or delays primary disease treatment. Some studies have evaluated the risk factors for cholecystitis after SEMS placement for MBO.1 Multiple reports have shown that covered SEMS (C-SEMS) and cystic duct obstruction by the tumor are risk factors for cholecystitis after SEMS placement for distal MBO (DMBO).3,4 However, the definitive risk factor remains unclear. The metal stents are a significant risk factor for cholecystitis in cases of cholecystitis after SEMS placement for hilar MBO (HMBO),5 and the rate of cholecystitis is higher in the unilateral stenting group than in the bilateral stenting group.6 Thus, the risk factors for cholecystitis after SEMS placement for both DMBO and HMBO remain unknown. Moreover, the risk factors and treatment methods for cholecystitis after SEMS placement have not been identified.
Although the standard treatment for acute cholecystitis (AC) is early cholecystectomy,7 high-risk surgical patients with AC are often initially treated with drainage, such as percutaneous transhepatic gallbladder drainage (PTGBD) or percutaneous transhepatic gallbladder aspiration (PTGBA).8 Although PTGBD is recognized as the standard drainage method for AC in the Tokyo Guidelines 2018 (TG2018), the appropriate drainage method for patients with AC who are unfit for surgery is unknown. PTGBD has some disadvantages compared to PTGBA, such as the requirement of catheter management and decreasing the quality of life of patients.9 Recently, there have been some reports on the efficacy of endoscopic ultrasonography-guided gallbladder drainage (EUS-GBD) in high-risk surgical patients with AC as an alternative treatment to PTGBD. One randomized controlled trial (RCT) indicated that EUS-GBD improved outcomes compared to PTGBD in patients who were not indicated for cholecystectomy.10 However, EUS-GBD is still performed only by expert endoscopists in high-volume centers. In contrast, PTGBA is an easy procedure and has a low complication rate; therefore, an alternative nonsurgical gallbladder drainage method is used in some high-risk surgical patients.8,9,11 In our hospital, PTGBA was chosen as the first treatment for high-risk surgical patients with AC because it does not require catheter management. However, the treatment outcomes of PTGBA in high-risk surgical patients with AC remain unclear.
The aim of this study was to clarify the risk factors for AC after SEMS placement, as well as the predictive factors of PTGBA as a treatment for AC after biliary SEMS placement.
This was a single-center, retrospective, observational cohort study conducted at the Kyushu Medical Hospital. We reviewed 529 patients who underwent endoscopic retrograde cholangiopancreatography (ERCP) with SEMS placement for DMBO or HMBO due to tumor invasion who were unfit for surgery between April 2011 and March 2020. The exclusion criteria were as follows: lost to follow-up, unknown details of the indwelling metallic stents, previous cholecystectomy, resectable stage of cancer, and the presence of AC before ERCP. Demographic, clinical, and endoscopic data were collected from the medical records. All patients were followed up at the end of the study or until death. The final follow-up date was June 12, 2020.
Endoscopic biliary drainage was performed in all patients using covered or uncovered metallic stents 6 to 12 mm in diameter and 6 to 12 cm in length. The stent type was determined by each endoscopist, and all procedures were performed by experts or trainees under expert supervision. The patients were administered midazolam and pentazocine as sedatives. Prophylactic antibiotics were administered prior to ERCP in all patients. In addition to bleeding tendency or a previous sphincterotomy, the papilla was managed with endoscopic sphincterotomy (EST). In the HMBO group, the drainage area (unilateral or bilateral stenting) and drainage approach (partial stent-in-stent [pSIS] or side-by-side [SBS]) were determined at the endoscopist’s discretion. When SBS was performed, the bile duct diameter after stent placement in the analysis was considered as the total diameter of the stents placed in the common bile duct. For example, when two 8 mm stents were placed using SBS, the bile duct diameter used in the analysis was 16 mm.
All the procedures were performed under intravenous sedation and local anesthesia. All patients were administered antibiotics according to the physician’s discretion. Pentazocine was used as an analgesic. Antibiotics were administered prior to performing PTGBA, which was performed by transhepatic puncture of the gallbladder using a 16–21 G needle under ultrasound guidance; the needle was removed after the gallbladder contents were aspirated as much as possible (20–300 mL). All procedures were performed by expert gastroenterologists or trainees under expert supervision.
AC was diagnosed according to the TG2018.12 AC was determined when imaging findings of AC (including gallbladder distention and gallbladder wall thickening) were accompanied by local signs of inflammation, including Murphy’s sign or right upper abdominal quadrant mass, pain, tenderness, and systemic signs of inflammation, including fever, elevated C-reactive protein, and elevated white blood cell count. Imaging findings were evaluated using transabdominal ultrasound (US) or computed tomography (CT). The grading of severity was as follows: mild, moderate, and severe according to the TG18/TG13 severity grading for AC.12,13
We evaluated the presence of tumor invasion to the cystic duct using imaging modalities, including endoscopic retrograde cholangiography, intraductal US, CT, magnetic resonance cholangiopancreatography, and EUS. Cystic duct obstruction was diagnosed when the tumor extending around the cystic duct was confirmed by any of the above imaging tests or when the cystic duct branched from an irregular narrowing of the bile duct or was not contrasted by ERCP.
The primary outcomes of this study were the risk factors for AC after SEMS placement and poor response factors for PTGBA. The secondary outcomes included the incidence of AC after SEMS placement and therapeutic efficacy and adverse events of PTGBA in this condition. The following variables were included in the analysis of risk factors for AC after SEMS placement: (1) cancer type (pancreatic cancer or non-pancreatic cancer), (2) treatment for cancer (treatment or best supportive care), (3) previous biliary stenting including endoscopic biliary stenting and endoscopic nasobiliary drainage, (4) location of the biliary stricture (hilar or distal bile duct), (5) cystic duct invasion by the tumor, (6) stent type (covered stent or uncovered stent), (7) bile duct diameter after stent placement (≤8 mm or ≥10 mm); (8) gallbladder stone, (9) acute cholangitis on ERCP, (10) stent length (>6 cm or ≤6 cm), and (11) EST. The following variables were included in the analysis of predictive factors for poor response to PTGBA: (1) to (9), (12) severity of AC (mild or moderate/severe), and (13) bile culture (positive or negative).
The outcomes of PTGBA were as follows: technical success was defined as sufficient aspiration of gallbladder contents, and clinical effectiveness was defined as the same definition for good response to treatment. We defined a good response to PTGBA as an improvement of at least two or three clinical outcomes of AC (i.e., improvement of fever, abdominal pain, and leukocytosis) without recurrence for at least 30 days. The good response group included patients with clinical improvement by one-time PTGBA, whereas patients who needed more than twice of PTGBA were categorized into the poor response group. Adverse events were defined as any procedure-related adverse events, including fever, abdominal pain, bleeding, bile peritonitis, and bile duct injury, within 2 weeks.
The Student t-test or the Mann-Whitney U-test was used for continuous variables, and the chi-square test or Fisher exact test was used for categorical variables. Univariate and multivariate logistic regression models were used to identify the risk factors for AC and poor response factors of PTGBA. Variables that were significantly different in the univariate model were entered into multivariate logistic regression models. Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for all variables. Differences were considered statistically significant at p<0.05. All statistical analyses were conducted using JMP 16 (SAS Institute Inc., Cary, NC, USA).
A total of 401 patients were retrospectively analyzed; 43 patients developed AC (AC group) and 358 patients did not develop AC (non-AC group) as a complication. Among 43 patients (AC group), 37 underwent PTGBA as the first treatment. We divided these patients into two groups according to their responses to PTGBA: good response (20 patients) and poor response (17 patients) groups. A flowchart of the enrolled patients is shown in Figure 1.
The characteristics of the 401 patients analyzed are presented in Table 1. The incidence of AC was 10.7% (43/401). AC and non-AC group patients showed no differences in age, sex, alcohol consumption, smoking, disease, or treatment for other diseases. The proportions of hilum bile duct stricture, cystic duct obstruction, and C-SEMS were significantly higher in the patients of AC group than in those of non-AC group (p=0.022, p<0.001, and p=0.001, respectively).
Among 43 patients, six patients underwent EUS-GBD and 37 underwent PTGBA (Fig. 1) as the initial treatment. The characteristics of the 37 patients are shown in Table 2. There were no differences in age, sex, alcohol consumption, smoking, disease, or treatment for diseases between the patients of good and poor response groups. The proportion of cystic duct obstruction, uncovered SEMS (U-SEMS), and positive bile culture were significantly higher in the patients of poor response group than those of the patients in the good response group (p=0.001, p=0.001, and p=0.032, respectively). As shown in Table 3, the technical success rate was 100% (37/37), and the clinical success rate was 54% (20/37). Adverse events occurred in two patients. Two patients had hemobilia, which was alleviated by conservative treatment.
The results of the univariate and multivariate analyses of risk factors for AC after SEMS placement are shown in Table 4. Univariate analysis showed that distal biliary stricture (OR, 2.46; 95% CI, 1.16–5.85; p=0.017), cystic duct obstruction (OR, 3.59; 95% CI, 1.89–6.94; p<0.001), and C-SEMS (OR, 3.08; 95% CI, 1.52–6.77; p=0.001) were predictors of AC after SEMS placement. In the multivariate logistic regression models, cystic duct obstruction (OR, 5.53; 95% CI, 2.76–11.3; p<0.001) and C-SEMS (OR, 4.98; 95% CI, 2.33–11.5; p<0.001) were independent predictive factors for AC.
In the univariate logistic regression models, cystic duct obstruction (OR, 9.75; 95% CI, 2.33–49.8; p=0.001), U-SEMS (OR, 10.3; 95% CI, 1.50–209; p=0.015), and positive bile culture (OR, 8.61; 95% CI, 1.29–171; p=0.023) were poor response factors of PTGBA for AC after SEMS placement. Multivariate analysis showed that cystic duct obstruction (OR, 12.4; 95% CI, 2.23–110; p=0.003) and U-SEMS (OR, 17.5; 95% CI, 1.81–474; p=0.011) were poor response factors of PTGBA for AC after SEMS placement (Table 5). The risk factor for the development of AC was C-SEMS, whereas the treatment poor response factor after onset was U-SEMS.
The present study demonstrated the risk factors for AC in patients with unresectable malignant biliary stricture and poor response factors of PTGBA for AC after SEMS placement. Cystic duct obstruction was a risk factor for AC and a poor response factor for PTGBA. With respect to stents, the C-SEMS group was a risk factor for AC. Conversely, patients with AC in the U-SEMS group showed a significantly poorer response to PTGBA than the C-SEMS group.
Previous studies reported several risk factors, including tumor invasion to the cystic duct, cholelithiasis, C-SEMS,14,15 and high axial force of SEMS16 as predictive factors of AC after SEMS placement.17-20 The main mechanism of AC may be narrowing or obstruction of the cystic duct due to tumor invasion and mechanical compression or tumor involvement of the feeding artery to the gallbladder.18 In the HMBO, a few studies showed that AC was significantly higher in SBS deployment than in pSIS deployment, and tumor progression to the cystic duct was also a risk factor.21-23 This mechanism of AC is considered as the blockage created by SEMS placement that leads to obstruction of the cystic duct. Furthermore, the mechanism of AC in patients with DMBO and HMBO is the same. In the present study, AC was present in 24 of 43 patients (55.8%) with tumor invasion to the cystic duct and in 33 of 43 patients (76%) with C-SEMS, with an OR of 5.53 (95% CI, 2.76–11.3; p<0.001) and 4.98 (95% CI, 2.33–11.5; p<0.001), respectively, suggesting that these were independent risk factors for AC.
Another novel result of our study was the poor treatment response factor for AC after SEMS placement. PTGBA is an easy procedure with a low complication rate. One RCT indicated that PTGBD was more effective than PTGBA for the treatment of AC.24 However, in high-risk surgical patients with AC, there are reports that PTGBA for AC after SEMS placement can be a useful alternative treatment method.8,9,11 Chopra et al.8 recommended PTGBA as the first treatment in high-risk surgical patients, and PTGBD should be reserved for the next procedure. Repetitive PTGBA might contribute to higher clinical success compared to PTGBD and endoscopic transpapillary gallbladder drainage.11,25 In our study, six of the 17 patients in the treatment poor response group could be improved by repeated PTGBA. Imai et al.9 reported that one-time PTGBA may be insufficient for patients with cystic duct obstruction. This study showed that the treatment of PTGBA response was significantly poor in the cystic duct obstruction group. With regard to the metallic stent, the U-SEMS group showed a poor response factor of PTGBA for AC. Theoretically, in the U-SEMS group, bile can pass through the mesh of the SEMS. However, AC occurred not only in the C-SEMS group but also in the U-SEMS stent group. Takinami et al.26 reported that tumor invasion and growth to the cystic duct were related to the onset of AC after U-SEMS deployment, and the U-SEMS group tended to develop AC later than its development in the C-SEMS group. In the present study, the median time from SEMS placement to the onset of AC was 39.2 days (range, 2–402 days) in C-SEMS and 165.7 days (range, 4–747 days) in U-SEMS, which tended to be longer in U-SEMS (p=0.028) (Fig. 2). We speculate that the pathogenic mechanism of AC in the U-SEMS group is closely related to not only stent placement but also tumor ingrowth, tumor invasion, and hyperplasia of the cystic duct, namely cystic duct obstruction, is strongly associated with the development of AC. Therefore, treatment with PTGBA appeared to be poor in the U-SEMS group. However, in the C-SEMS group without cystic duct obstruction, the clinical success rate of PTGBA was as high as 87.5% (14/16). Considering the aforementioned results, we propose a strategy for the treatment of high-risk surgical patients with AC that PTGBA may be recommended for patients with C-SEMS placement and without cystic duct obstruction, and treatment other than PTGBA may be recommended for patients with U-SEMS placement (Fig. 3).
The present study had certain limitations. First, this was a single-center retrospective study, thus, incomplete data and potential selection biases could not be avoided. Second, the sample size of patients with AC was small, and both DMBO and HMBO were used for evaluation. In particular, the number of SEMS in the HMBO group was relatively low, and the incidence of AC tended to be lower in the HMBO group than that of the DMBO group. This discrepancy might be statistically significant if a study with a larger number of patients was performed. Third, we used PTGBA, which has not yet been established. Further large-scale, multi-institutional, prospective studies are required to validate our results.
In conclusion, we should recognize the difference between the onset factor and poor response to treatment for AC after SEMS placement. After SEMS placement, the AC was initially treated with a single or repetitive PTGBA. However, in the case of U-SEMS, it may be better to consider the drainage method with immediate placement of the catheter.
Notes
Author Contributions
Conceptualization: AO, NF, TK, MH; Data curation: AO, TK, MH; Formal analysis: AO, TK; Investigation: AO, NF, TK, MH; Methodology: AO, NF, TK, MH; Project administration: AO, TK; Resources: AO, TK; Software: AO, TK; Supervision: NF, TK; Validation: NF, TK; Visualization: AO; Writing–original draft: AO, NF, TK; Writing–review & editing: all authors.
REFERENCES
1. Isayama H, Komatsu Y, Tsujino T, et al. A prospective randomised study of “covered” versus “uncovered” diamond stents for the management of distal malignant biliary obstruction. Gut. 2004; 53:729–734.

2. Fumex F, Coumaros D, Napoleon B, et al. Similar performance but higher cholecystitis rate with covered biliary stents: results from a prospective multicenter evaluation. Endoscopy. 2006; 38:787–792.

3. Minami Y, Kudo M. Hepatocellular carcinoma with obstructive jaundice: endoscopic and percutaneous biliary drainage. Dig Dis. 2012; 30:592–597.

4. Kim GH, Ryoo SK, Park JK, et al. Risk factors for pancreatitis and cholecystitis after endoscopic biliary stenting in patients with malignant extrahepatic bile duct obstruction. Clin Endosc. 2019; 52:598–605.

5. Kanno Y, Koshita S, Ogawa T, et al. Inside plastic stents versus metal stents for treating unresectable malignant perihilar biliary obstructions: a retrospective comparative study. Clin Endosc. 2020; 53:735–742.

6. Lee TH, Kim TH, Moon JH, et al. Bilateral versus unilateral placement of metal stents for inoperable high-grade malignant hilar biliary strictures: a multicenter, prospective, randomized study (with video). Gastrointest Endosc. 2017; 86:817–827.

7. Huffman JL, Schenker S. Acute acalculous cholecystitis: a review. Clin Gastroenterol Hepatol. 2010; 8:15–22.

8. Chopra S, Dodd GD 3rd, Mumbower AL, et al. Treatment of acute cholecystitis in non-critically ill patients at high surgical risk: comparison of clinical outcomes after gallbladder aspiration and after percutaneous cholecystostomy. AJR Am J Roentgenol. 2001; 176:1025–1031.

9. Imai Y, Hasegawa T, Sato Y, et al. Management of acute cholecystitis after biliary stenting for malignant obstruction: comparison of percutaneous gallbladder drainage and aspiration. Jpn J Radiol. 2019; 37:719–726.

10. Teoh AYB, Kitano M, Itoi T, et al. Endosonography-guided gallbladder drainage versus percutaneous cholecystostomy in very high-risk surgical patients with acute cholecystitis: an international randomised multicentre controlled superiority trial (DRAC 1). Gut. 2020; 69:1085–1091.

11. Komatsu S, Tsuchida S, Tsukamoto T, et al. Current role of percutaneous transhepatic gallbladder aspiration: from palliative to curative management for acute cholecystitis. J Hepatobiliary Pancreat Sci. 2016; 23:708–714.

12. Yokoe M, Hata J, Takada T, et al. Tokyo Guidelines 2018: diagnostic criteria and severity grading of acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:41–54.
13. Yokoe M, Takada T, Strasberg SM, et al. New diagnostic criteria and severity assessment of acute cholecystitis in revised Tokyo Guidelines. J Hepatobiliary Pancreat Sci. 2012; 19:578–585.

14. Jang S, Stevens T, Parsi M, et al. Association of covered metallic stents with cholecystitis and stent migration in malignant biliary stricture. Gastrointest Endosc. 2018; 87:1061–1070.

15. Cao J, Peng C, Ding X, et al. Risk factors for post-ERCP cholecystitis: a single-center retrospective study. BMC Gastroenterol. 2018; 18:128.

16. Isayama H, Nakai Y, Toyokawa Y, et al. Measurement of radial and axial forces of biliary self-expandable metallic stents. Gastrointest Endosc. 2009; 70:37–44.

17. Shimizu S, Naitoh I, Nakazawa T, et al. Predictive factors for pancreatitis and cholecystitis in endoscopic covered metal stenting for distal malignant biliary obstruction. J Gastroenterol Hepatol. 2013; 28:68–72.

18. Nakai Y, Isayama H, Kawakubo K, et al. Metallic stent with high axial force as a risk factor for cholecystitis in distal malignant biliary obstruction. J Gastroenterol Hepatol. 2014; 29:1557–1562.

19. Isayama H, Kawabe T, Nakai Y, et al. Cholecystitis after metallic stent placement in patients with malignant distal biliary obstruction. Clin Gastroenterol Hepatol. 2006; 4:1148–1153.

20. Suk KT, Kim HS, Kim JW, et al. Risk factors for cholecystitis after metal stent placement in malignant biliary obstruction. Gastrointest Endosc. 2006; 64:522–529.

21. Fukasawa M, Takano S, Shindo H, et al. Endoscopic biliary stenting for unresectable malignant hilar obstruction. Clin J Gastroenterol. 2017; 10:485–490.

22. Naitoh I, Hayashi K, Nakazawa T, et al. Side-by-side versus stent-in-stent deployment in bilateral endoscopic metal stenting for malignant hilar biliary obstruction. Dig Dis Sci. 2012; 57:3279–3285.

23. Gwon DI, Ko GY, Yoon HK, et al. Prospective evaluation of a newly designed T-configured stent graft system for palliative treatment of advanced hilar malignant biliary obstructions. J Vasc Interv Radiol. 2010; 21:1410–1418.

24. Ito K, Fujita N, Noda Y, et al. Percutaneous cholecystostomy versus gallbladder aspiration for acute cholecystitis: a prospective randomized controlled trial. AJR Am J Roentgenol. 2004; 183:193–196.

Fig. 1.
Flow chart of patients enrolled in the study. SEMS, self-expandable metallic stent; AC, acute cholecystitis; ERCP, endoscopic retrograde cholangiopancreatography; EUS-GBD, endoscopic ultrasonography-guided gallbladder drainage; PTGBA, percutaneous transhepatic gallbladder aspiration; PTGBD, percutaneous transhepatic gallbladder drainage; ETGBD, endoscopic transpapillary gallbladder drainage.
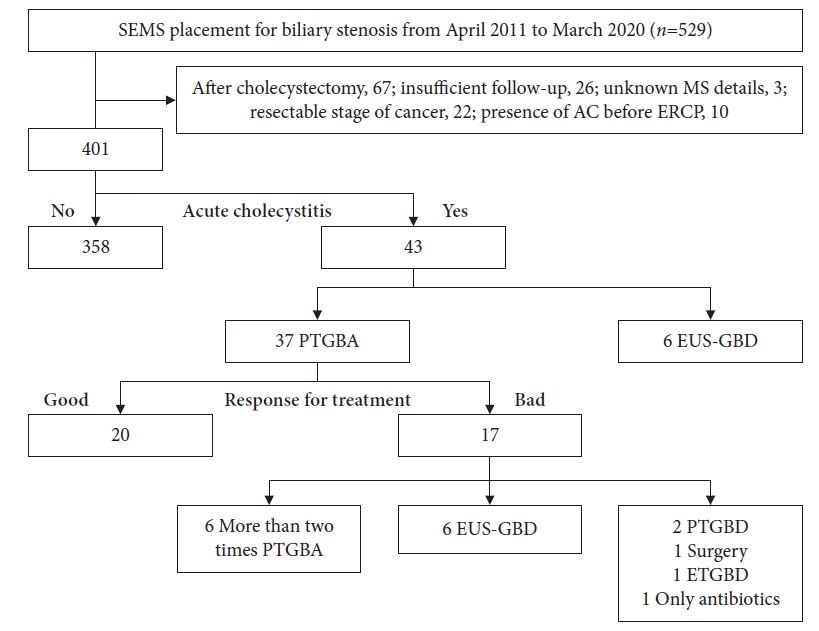
Fig. 2.
Comparison with the median time from self-expandable metallic stent (SEMS) placement to the onset of acute cholecystitis between uncovered (U)-SEMS and covered (C)-SEMS groups.
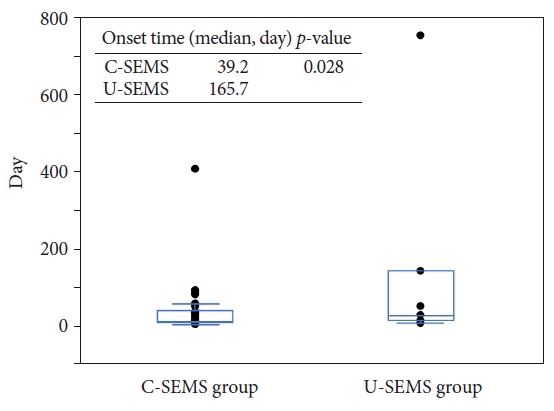
Fig. 3.
Treatment strategy for high-risk surgical patients with acute cholecystitis after biliary metallic stent. PTGBA, percutaneous transhepatic gallbladder aspiration; PTGBD, percutaneous transhepatic gallbladder drainage; EUS-GBD, endoscopic ultrasonography-guided gallbladder drainage.
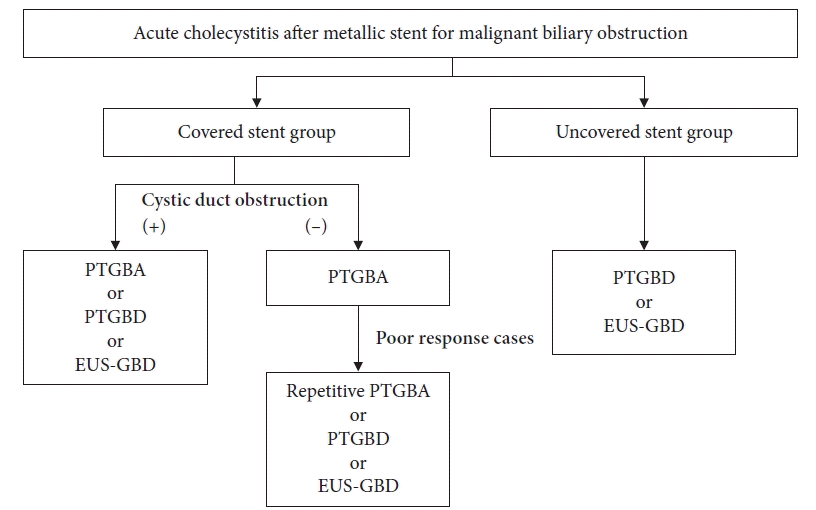
Table 1.
Baseline characteristics of patients who underwent metallic stent placement
Table 2.
Baseline patient characteristics of acute cholecystitis group who underwent percutaneous transhepatic aspiration
Table 3.
Clinical outcomes of PTGBA for acute cholecystitis after metallic stent placement (n=37)
| Variable | PTGBA |
|---|---|
| Technical success | 37 (100) |
| Clinical success | 20 (54) |
| Adverse event (hemobilia) | 2 (5.4) |
| Survival time (day) | 339 (53–2,140) |
Table 4.
Risk factors for acute cholecystitis after metallic stent placement in univariate and multivariate logistic regression analyses
Table 5.
Poor response factors of percutaneous gallbladder aspiration for acute cholecystitis after metallic stent placement in univariate and multivariate logistic regression analyses




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download