Introduction
Idiopathic nephrotic syndrome (NS), clinically characterized by massive proteinuria, hypoalbuminemia, hyperlipidemia, and generalized edema, is a hypercoagulable state in which children are at risk of venous thromboembolism [
1]. Although the difference in incidence between children and adults is still to be elucidated, the risk of thromboembolic phenomenon is estimated to be 1.8%–5% in children with NS [
2], whereas its incidence in adult patients is approximately 25% [
1]. Patients with severe proteinuria have a 3.4-fold higher risk of thromboembolism [
3]. Notably, a higher risk has also been reported in children with steroid-resistant NS than in those with steroid-sensitive NS [
4].
In the 19th century, renal vein thrombosis was recognized for the first time to be associated with NS [
1]. This observation was polished with the association between NS and venous thrombosis, as reported by Derow et al. in 1939 [
5]. Since that time, it has been realized that thromboembolism may arise in patients with NS and form in either arteries or veins, including pulmonary embolism, renal vein thrombosis, and cerebral venous sinus thrombosis (CVST).
However, the majority of thrombotic events result from a venous origin. The occurrence of inferior vena cava thrombosis, renal vein thrombosis, or pulmonary embolism, is frequent, while CVST is less common [
6]. Although CVST is very rare in children, it has been reported most commonly in the cerebral sagittal sinus [
7]. The mortality rate of CVST is approximately 10% and generally results from cerebral herniation in the acute phase and an underlying disorder in the chronic phase [
8]. Moreover, 6% to 10% of patients who survive have severe residual neurological sequelae despite therapeutic intervention [
9]. As CVST is one of the major life-threatening complications in NS, understanding CVST, immediate anticoagulation, and treatment is critical in children with NS with thrombogenic predisposition. Here, we present a pediatric patient with steroid-dependent NS (SDNS) and acute CVST with a favorable outcome.
Case report
A 3-year and 6-month-old boy visited the emergency department because of generalized edema and abdominal pain. He had swelling on both eyelids and poor oral intake and was lethargic for 3 days, followed by the appearance and worsening of whole body edema and abdominal pain before he was admitted. He had a family history of paternal diabetes mellitus and hypothyroidism. He also had a past medical history of NS treated at another hospital before he came to our hospital. He was suspected of having subclinical hypothyroidism and was followed up without medication. On physical examination, pretibial pitting edema on both legs and abdominal distention were observed.
Laboratory investigation revealed massive proteinuria (spot urinary protein-to-creatinine ratio [UPCR] of 26 g/g, normal <0.2 g/g), low serum albumin level (1.6 g/dL), and hypercholesterolemia (369 mg/dL). His renal function was within normal limits. The thyroid function test revealed an elevated serum thyroid-stimulating hormone level (32.3 μIU/mL) and normal T3 (1.01 ng/mL) and free T4 (1.08 ng/dL) levels. Twenty-four-hour urine protein and creatinine excretion were measured as 3,683 mg and 180 mg, respectively, with a total urinary volume of 540 mL/day. The UPCR gradually increased to 30.7 g/g in spot urine. Levels of complement components C3 and C4 were all within the normal ranges (98 mg/dL and 23.2 mg/dL, respectively). The antistreptolysin O titer was 85 IU/mL (reference range, 0-200 IU/mL). The serologic tests for antinuclear antibodies, antineutrophil cytoplasmic antibodies, and anti-double-stranded DNA were all negative. He was then treated with deflazacort (2 mg/kg/day), albumin replacement and diuretics, and an angiotensin-converting enzyme inhibitor. The UPCR decreased to 7.7 g/g during the 2-week treatment period before he was discharged with oral medication, which was still a high amount of protein in the urine.
After 5 days of discharge, the boy presented to our emergency room with a 3-day history of aggravating headaches and a four-time history of vomiting after eating. On the day of presentation, he was not able to stand and even speak but had no fever or history of seizures. His vital signs were as follows: high blood pressure, 98/64 mmHg; pulse rate, 135 beats/min (tachycardia); and a slightly decreased respiratory rate of 20 breaths/min. Physical examination showed an acute ill-looking appearance, drowsy mental status, and pretibial pitting edema on both of his lower legs, gaining body weight from 15.6 to 17.0 kg.
The initial coagulation function test revealed an elevated serum fibrinogen level of 468 mg/dL (reference value, 200–400 mg/dL), increased D-dimer level of 1.1 μg/mL (fibrinogen equivalent units, 0–0.5 μg/mL), and decreased antithrombin III (AT-III) level of 55% (80%–120%). The levels of fibrinogen, D-dimer, and AT-III were changed to 680 mg/dL, 10.1 μg/mL, and 41%, respectively, on day 7 (
Table 1). The activated partial thromboplastin time (aPTT), prothrombin time, and international normalized ratio (INR) were all within normal limits. The UPCR increased to 23.0 g/g again, showing heavy proteinuria with a significantly low albumin level of 1.8 g/dL.
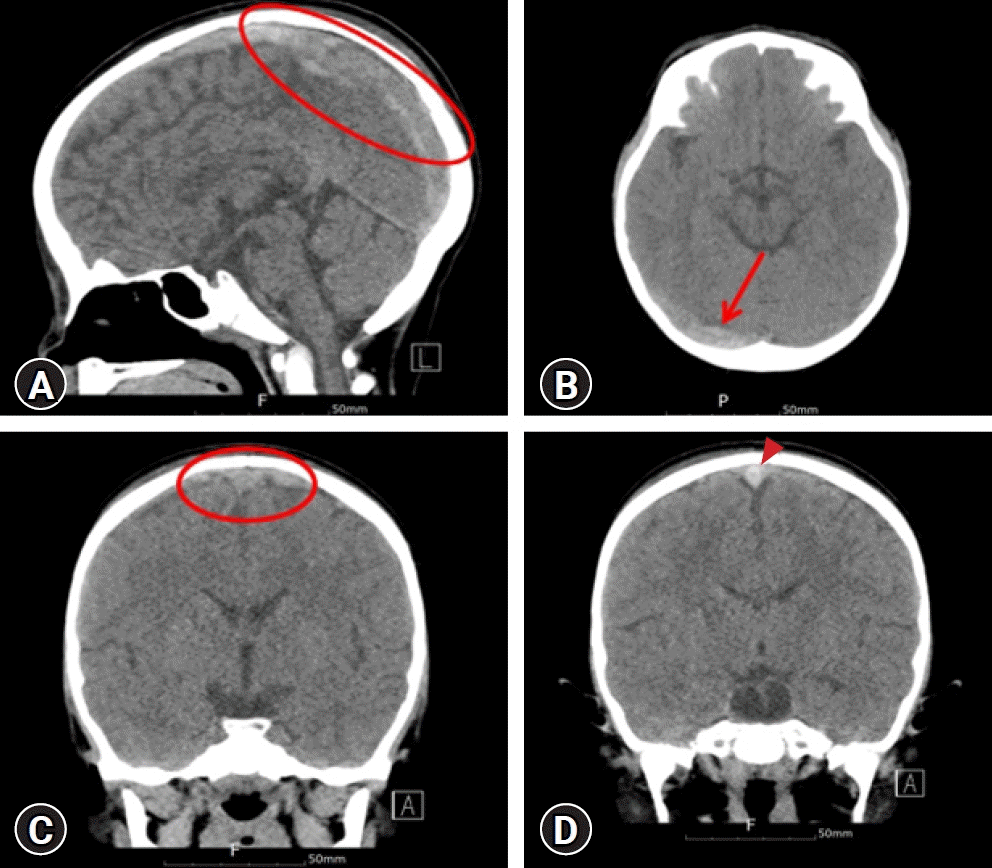
On admission, non-contrast computed tomography (CT) of the brain revealed a hyperintense prominence and segmentally increased attenuation of the superior sagittal, right transverse, and sigmoid sinuses with slightly engorged cortical veins, suggesting acute dural venous sinus thrombosis (
Fig. 1). A coronal non-enhanced CT image of the patient showed an area of central hyperattenuation in the superior sagittal sinus (
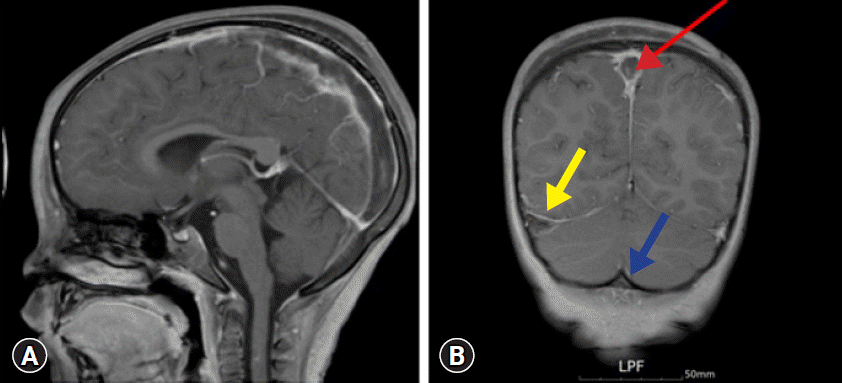
Fig. 1). Magnetic resonance imaging (MRI) also demonstrated empty delta signs, contrast outlining a filling defect due to a thrombus (
Fig. 2). No cerebral infarction or hemorrhage was observed.
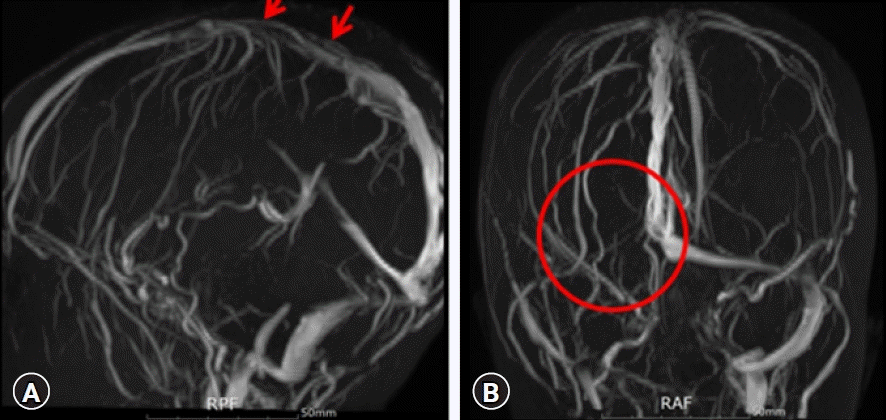
The patient was started on 100 U/kg of unfractionated heparin (UFH) intravenously at loading and subsequent doses of 25 U/kg/hr with a target goal of an aPTT of 60 to 80 seconds. UFH was switched to enoxaparin, low-molecular-weight heparin. A vitamin K antagonist (warfarin) was also added to the patient at a dose of 0.1 to 0.2 mg/kg per oral a day for anticoagulation, planning to be used a minimum duration of 3 months up to 6 months. Three weeks later, radiological assessment for recanalization was performed with magnetic resonance venography (MRV), resulting in substantial obliteration and slight residual dural sinus thrombosis in the superior sagittal sinus (
Fig. 3).
Discussion
The current report describes CVST in a child with SDNS, initially diagnosed by contrast non-enhanced CT and consecutively confirmed by contrast-enhanced MRI, followed by MRV for improvement. Although we have theoretically acknowledged the occurrence of thrombosis in patients with NS, to the best of our knowledge, this is the rare case of CVST reported in a pediatric patient aged <5 years with NS in Korea. This specific case of CVST contributes to the spectrum of serious complications in younger children with SDNS. Additionally, it can influence the early detection and prompt management of children with CVST.
According to a review of the literature, hypercoagulability induced by severe proteinuria, hypoalbuminemia, and hypovolemia, using diuretics, increased synthesis of fibrinogen and other clotting factors (factor V, factor VIII, von Willebrand factor, α2-plasmin inhibitor, and plasminogen activator inhibitor) [
1], decreased endogenous antithrombotic factors (protein C, protein S, AT-III, and tissue factor pathway inhibitor), accelerated generation of thromboplastin, thrombocytosis, platelet aggregation, and activation of the coagulation system, work together to produce thrombogenic conditions in NS [
10,
11]. Bacterial peritonitis and sepsis can also induce systemic thrombosis triggered by microbial endotoxins or exotoxins and subsequently release inflammatory cytokines from monocytes in NS [
11]. Inflammatory responses accompanying immune injury in the intraglomerular microenvironment may produce procoagulants and strengthen the expression of molecules that reduce fibrinolysis [
10]. Moreover, hemostatic abnormalities mediated by the responses of tissue factor to tumor necrosis factor, interleukin-1, and interleukin-6 at the cell surface can cause hypercoagulability [
12]. In contrast, low plasma levels of thyroid hormone lead to a hypocoagulable and hyperfibrinolytic state, whereas high levels of thyroid hormone increase the risk of venous thromboembolism.
In the present case, the patient did not show any infective features or laboratory results. However, it was thought that urinary loss of the endogenous anticoagulant AT-III and the thrombophilic state as a result of altered permselectivity of the glomerular basement membrane contributed to the acute dural venous sinus thrombosis that was made with respect to SDNS [
10]. Hyperfibrinogenemia is a reflection of increased acute-phase reactants to hypoalbuminemia in patients [
11]. The increase in the fibrinogen level is believed to promote platelet and erythrocyte aggregation and blood viscosity for fibrin formation.
Like in the patient, dural venous sinus thrombosis in NS presents with symptoms including headache, vomiting, papilledema, unilateral motor weakness, speech disturbance, seizures, and mental status change. Non-enhanced CT scans are frequently normal and require contrast CT or MRI. A triangular area of enhancement with a relatively low-attenuating center is the thrombosed sinus on contrast-enhanced CT or MRI, showing an empty delta sign, which is a filling defect in the dural venous sinus [
13]. MRV is regarded as the gold standard imaging technique for CVST, with persistent hyperintense thrombus signal on T1WI and T2WI until the 15th day [
13]. In our patient, MRV was performed to confirm recanalization and improvement without hemorrhage on the 3rd week.
Regarding thrombosis treatment, what is the first choice, when acute CVST complications occur, and how we evaluate the efficacy of anticoagulation therapy are not clearly established in patients with NS because of the lack of large randomized trials and guidelines. Conventional anticoagulation remains the standard therapy in the presence of thrombosis. Currently, anticoagulants fall into two categories: (1) parenteral anticoagulants such as heparin and low-molecular-weight heparin, which bind to and potentiate the effects of AT-III, and (2) oral anticoagulants such as vitamin K antagonists and direct-acting oral anticoagulants (factor Xa and IIa inhibitors) [
14].
Anticoagulant therapy with heparin and thrombolytic therapy with urokinase is an important and invaluable therapy for CVST. Although the use of UFH may be associated with intracerebral hemorrhage, UFH has the benefit that the effect can be rapidly adjusted when severe complications occur or surgical intervention is required [
15]. In the case reported here, we initially administered UFH with a target goal of an aPTT of 60 to 80 seconds and switched to enoxaparin because of its practical advantages, which do not require routine monitoring and dosage adjustment based on coagulation times, together with warfarin with an INR of between 2 and 3 [
14].
The normalization of albumin levels is essential as it lowers hypercoagulability and the levels of other clotting factors, restoring the balance of prothrombotic and antithrombotic factors. Decreased albumin levels in patients with NS can lead to a poor response to anticoagulation treatment. Therefore, prompt treatment of NS plays a crucial role in the prevention and treatment of thrombosis. Intriguingly, AT-III transfusion with a molecular weight similar to that of albumin was effective in the present case. Recovery and maintenance of anticoagulation with normal AT-III activity induced the alleviation of thrombosis before the serum albumin levels were increased in our patient. This indicates that AT-III may be a sensitive biomarker to prevent further thrombus formation in patients with NS and predict therapeutic effectiveness, in addition to anticoagulation therapy with heparin, improvement of urinary protein loss, and correction of intravascular volume depletion.
In conclusion, patients with NS may develop CVST that can lead to life-threatening outcomes, particularly in the presence of a prothrombotic state, depending on the disease severity. Imaging studies such as non-contrast CT or MRI are useful for early detection and diagnosis, although MRV is the gold standard tool for diagnosing CVST. Anticoagulation therapy plays a key role in treating and preventing acute and recurrent thrombosis. The present case showed a good response to anticoagulation therapy, together with AT-III. However, appropriate type, dosing, and monitoring of anticoagulation require further clinical trials to improve our understanding and knowledge of the optimal prevention and management strategies in younger children with NS.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download