Abstract
Research using experimental animals has substantially contributed to advances in science and medicine. Animal experiments are nearly essential for biomedical research and development efforts. Because many animals are sacrificed, researchers should consider the welfare of experimental animals and related ethical issues, along with the successful results of their experiments. This review introduces the criteria that should be considered in terms of experimental animal ethics, based on the principles of the 3 R’s: replacement, representing careful consideration of the need for animal experiments; reduction, representing the use of the minimal number of animals to obtain meaningful experimental results; and refinement, representing continuous effects to find alternative methods to reduce pain and distress in experimental animals. Based on these principles, the following points should be considered when planning experiments: the necessity of animal experiments; alternatives to animal experiments; the relevance of the species and numbers of experimental animals; appropriate assessment and management of pain; the proper usage of sedatives, painkillers, and anesthesia; and valid timing for humane endpoints and euthanasia. These criteria are beneficial for both experimental animals and researchers because careful handling to ensure experimental animal welfare guarantees that scientific research will yield convincing, repeatable, and accurate results.
Go to : 
In vivo experiments using animal models have substantially contributed to scientific and medical advances, and they continue to have an important role in research. Thus, there is a need to consider the ethical use and welfare of animals in scientific experiments. Experimental animals are considered living reagents in terms of reliability, reproducibility, and stability. Animal research is based on the principles of the 3 Rs published in 1959, which emphasize ethics, cost, and efficiency: (1) replacement, representing careful consideration of the need for animal experiments; (2) reduction, representing use of the minimal number of animals to obtain meaningful experimental results; and (3) refinement, representing animal welfare [1].
Because the use of experimental animals is increasing with developments in science and industry, experimental animal ethics systems have been adopted to prevent reckless animal experiments and improve the welfare, scientific use, and ethical treatment of experimental animals. Multiple factors have led to changes in animal experimentation, including the requirement to demonstrate the legitimacy of animal research, diversification of animal experiments, strengthening of the legal system with regard to animal research and experimental animals, and activities of animal welfare organizations.
Experimental animals are living non-human vertebrate animals from zebrafish to non-human primates, which have developed nervous systems that can feel pain and have consciousness [2]. Because animal experiments can cause pain and distress, animals should not be killed, injured, or made to suffer unnecessarily. The ethical use of animals and animal welfare are important aspects of the scientific use of experimental animals. This review focuses on the welfare and ethical use of experimental animals.
Go to : 
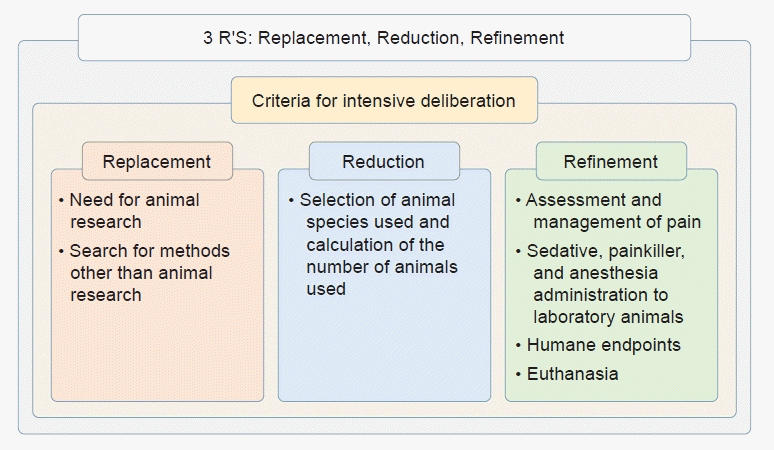
The annual global use of non-human vertebrate animals was estimated to be 192.1 million in 2015 [2]. Furthermore, the number of experimental animals used in 2021 in the Republic of Korea was 4.88 million, with a mean of 13,000 animals used in experiments each day (www.animal.go.kr). The regulation of ethical aspects of animal research varies according to multiple factors [3]. The use of animals in research is a privilege given by society to research organizations in the hope of providing meaningful new knowledge or improving human and animal welfare. The Institute of Laboratory Animal Resources Guide for the Care and Use of Laboratory Animals describes the minimum ethical requirements for animal research. Researchers who use experimental animals are required to submit a plan for animal research to their Institutional Animal Care and Use Committee (IACUC) for approval. The criteria for consideration of the animal research plan consist of nine categories: Principles of the 3 Rs; Criteria for intensive deliberation; Need for animal research; Search for methods other than animal research; Selection of animal species used and calculation of the number of animals used; Assessment and management of pain; Sedative, painkiller, and anesthesia administration to laboratory animals; Humane endpoints; Euthanasia (Fig. 1).
All animal experiments should be conducted in compliance with these principles [1].
Alternative animal research is composed of absolute replacement and relative replacement. Absolute replacement involves computer simulation and an inanimate system; relative replacement refers to in vitro experiments, such as cell and/or tissue culture, as well as the use of phylogenetically lower species (i.e., insects or other invertebrates).
Multiple methods are available to reduce the number of animals used as much as possible, including rational choice of experimental groups, choice of most appropriate experimental animal model, accurate statistical analysis, and minimization of unnecessary loss by meticulous animal care.
There is a need to prevent unnecessary pain in experimental animals by optimizing experimental methods and by using appropriate techniques and facilities. In animal research, the refinement of methods to lessen or eliminate unnecessary pain, distress, and suffering in experimental animals is the most commonly implemented principle of the 3 Rs. Furthermore, refinement improves animal welfare, which affects the quality of animal research [4].
Two other Rs (relevance and avoidance of redundancy) should also be considered. Relevance refers to an animal-based research plan that should be able to provide solutions to problems with important scientific, medical, and/or social implications. The benefits to humans and animals through animal experiments should outweigh all risks and damage done to animals. The avoidance of redundancy refers to the need to avoid duplicate research in animal experiments through exhaustive literature analysis to confirm that similar experiments were not conducted previously [5].
When planning an animal experiment, researchers should fully consider ethical issues. The following criteria should be assessed when the IACUC evaluates plans for animal experiments (Table 1).
Criteria for intensive deliberation
• The need for animal experiments—the conclusion of the research cannot be obtained without performing animal experiments.
• Whether a search has been conducted for alternatives to animal experiments—researchers should strive to identify other approaches for their research that do not involve animal experiments (e.g., using invertebrate models, in vitro systems, or in silico methods).
• Whether animal welfare and ethical treatment are appropriate in terms of animal experiments and animal management.
• Whether the experimental animal species and number of animals are appropriate for the research purpose.
• Whether the degree of pain and distress experienced by experimental animals during experiments is acceptable.
• Whether the method of euthanasia for experimental animals is appropriate and the timing of humanitarian termination is reasonable.
• Whether appropriate measures are planned to reduce pain in experimental animals during experiments.
• Whether the research is in compliance with the provisions of Article 24 of the Animal Protection Act (Prohibition of Animal Testing).
• Whether experimental animal handling is conducted in an ethical manner, and whether the research staff have appropriate knowledge and training related to animal experiments.
• Other matters considered necessary by the Committee for the Protection and Ethical Handling of Experimental Animals.
The first consideration when designing animal experiments is to establish the need for animal experimentation. Specifically, animal experiments should be conducted only when the research purpose cannot be achieved without the use of animals. Then, a harm-benefit analysis should be conducted to determine whether the scientific and social benefits of animal use outweigh the harm of pain and damage to the experimental animals. To extensively investigate the literature concerning the need for animal experiments, experts in relevant research fields should be consulted. Discussions among IACUC members should focus on the purpose of and need for animal experiments, rather than the scientific benefits of the research. Therefore, researchers should prepare plans for animal experiments in simple terms to ensure that IACUC members understand the purpose of and need for animal experiments. Additionally, alternative experimental technologies are available in the life sciences, such as mathematical modeling, computer simulations, cell units, and invertebrate models (e.g., Drosophila).
Researchers should consider alternative methods beginning during the initial stage of planning an animal experiment, particularly when the experiment is likely to cause pain. The IACUC requires researchers to perform a database search to find alternative methods; the results of the search should be included in the experimental plan. The name(s) of the database(s) searched should be included in the experimental plan, along with the date and time of the search. Generally, multiple databases should be searched. Details of the search strategy (e.g., appropriate scientific terms) should be included in the experimental plan. The researcher should be asked to provide reasons why alternative methods cannot be used, if the committee has identified appropriate alternative methods through a database search. To facilitate an efficient search for alternative methods, researchers should clearly describe the information that they wish to obtain from animal experiments. To obtain information regarding alternatives to animal experiments, a systematic search can be conducted at DB-ALM of the European Commission’s EURL ECVAM [6] or at Animal Alt-ZEBET of the Federal Institute for Risk Assessment [7].
In animal experiments, the use of experimentally bred animals should be prioritized; the direct use of pets or wild animals should be avoided. If wild animals are used, the impact of their capture on the ecosystem must be fully considered. Experiments involving specific wild animals, including monkeys, should be conducted after careful discussion regarding their handling.
A key variable to consider in animal experiments is selection of the most appropriate animal species. Animal species should be selected by referring to the literature or expert opinions, according to the purpose and content of the experiment. If the purpose of an animal experiment is to explore biological principles, an animal species with specific characteristics should be selected. For example, the naked mole-rat (Heterocephalus glaber) is an emerging animal model in aging studies because it is the longest-lived rodent that does not exhibit age-dependent degenerative diseases for 80% of its lifespan [8]. The fruit fly (Drosophila melanogaster) is also a popular system in which to study the associations of longevity with genetic modifications [9,10]; this insect has a short lifespan and is an appropriate system in which to modify specific genes. These characteristics are helpful in experiments regarding the contributions of specific genes to longevity. Additionally, the use of D. melanogaster is free of ethical concerns because it is not a vertebrate animal model system. H. glaber is appropriate for comparative research to determine the mechanisms of longevity [11], whereas D. melanogaster is appropriate for studies of the effects of specific genes on longevity [12-14]. Therefore, the purpose of the research is a major factor in animal species selection. In many instances, specific methodologies have already been established to identify appropriate animal species for experiments focused on diagnostic material development, raw drug material development, or safety tests [15,16]. This decision should be based on the literature or consultation with experts. For education, the selection of animal species should be made based on handling convenience and potential educational benefits.
The number of animals is important when designing animal experiments. Although research performed with insufficient numbers of experimental animals cannot yield significant results, the use of excessive numbers of animals may lead to criticism regarding ethical issues, as well as unnecessary financial expenditures and labor costs. Although it is often difficult to determine the ideal number of animals for use in experiments, statistical methods can help to estimate the approximate number of animals required for a specific study. Relevant statistical evidence can also be obtained from similar experiments in previous reports or from preliminary experiments. Additionally, researchers should check whether the estimated number of animals includes a sufficient number to compensate for loss caused by unexpected deaths and other events that may occur during experiments. However, for situations where a specific dropout rate cannot be determined in preliminary experiments, an adequate number is generally regarded as approximately 10% of the minimum number of animals for the experiment.
Because animal experiments can cause pain and distress, animals should not be killed, injured, or made to suffer unnecessarily. Everyone involved in the use of animals in research is responsible for alleviating or eliminating the suffering of experimental animals. Animals should only be handled by trained personnel with a full understanding of the animals’ habits, physiology, anatomy, and appropriate environment. Researchers should have a comprehensive understanding of their legal requirements; be able to identify pain in animals, and appropriately eliminate or mitigate such pain; and establish a humanitarian endpoint to the experiment. Experiments should be carried out within the shortest possible interval, and the number of animals should be minimized Additionally, the phylogenetically lowest animal species should be selected from among species appropriate for each experiment. Because pain and distress are likely to substantially alter an animal’s physiological state, these factors should be avoided to minimize interference with research activities and bias in the experimental results. Researchers should be able to identify indicators of pain in the animal species used in their experiments, primarily by monitoring changes in body weight, body condition score, physical appearance, responses to external stimuli, and behavior.
Appropriate animal handling and anesthesia administration to experimental animals are essential in terms of animal welfare, as well as the acquisition of reproducible and reliable experimental results. Proper handling significantly reduces pain and discomfort to animals, thereby facilitating experimental manipulation and preventing risks to experimenters. Appropriate anesthesia methods (intravenous, intraperitoneal, intramuscular, subcutaneous, and peroral) should be selected for the purpose of each study (e.g., narcosis, relaxation, and/or analgesia); most anesthetics are classified as psychotropic drugs (pentobarbital, ketamine, and propofol). The representative anesthetics in animal experiments are barbiturate (pentobarbital sodium and thiopental sodium), dissociative anesthetics (Ketamine HCl and Zoletil), hypnotics (avertin and propofol), diethyl ether, and isoflurane. Medications related to veterinary treatment, such as sedation, pain relief, and pre-anesthesia administration are atropine sulfate, xylazine, vecuronium bromide, and ibuprofen. It is important to choose an appropriate anesthetic because its efficacy varies according to species, age, sex, size, and health condition.
The establishment of humane endpoints is important for mitigating extreme distress and pain in experimental animals during experiments. Even if an experiment is not expected to cause animals distress and/or pain, there is a need to plan for the management of unexpected animal suffering and determine when to end the experiment. The International Council of Laboratory Animal Science published 10 basic principles for the establishment of humane endpoints in 2006 [17]. The ideal humane endpoint is completion of the experiment before the animal experience distress, pain, or suffering. Furthermore, researchers should not use the death of an animal as the criterion for a humane endpoint.
Euthanasia involves loss of consciousness within a short time to avoid suffering; death is the final result. The following considerations are important with respect to euthanasia: the method must not involve pain, the loss of consciousness must occur within a short period of time, the time to death must be short, the method must always result in death, the method must be safe for researchers and appropriate for the purpose of the study, and the method must be economical and appropriate for histopathological evaluation. The potency and side effects of the euthanasia method must also be considered. Because improper euthanasia is a violation of animal welfare regulations, researchers should refer to the American Veterinary Medical Association Guidelines for the Euthanasia of Animals [18].
Go to : 
Although animal experiments have contributed to advances in medical science, minimal consideration was initially given to the welfare of the experimental animals. However, there is increasing awareness of the importance of experimental animal welfare. Furthermore, animal experiments have become diversified and legal systems for animal experiments have been strengthened; doubts have been raised regarding the need for animal experiments. Before animal experiments can begin, the plans are evaluated by an IACUC using the criteria outlined in this review. The maintenance of experimental animal welfare is beneficial to both animals and researchers in terms of ensuring the acquisition of consistent, reliable, repeatable, and convincing results; unethical conditions could cause pain and distress, thereby distorting the animals’ responses and leading to bias in the experimental results. Therefore, animal experimentation must be performed in accordance with the ethical guidelines outlined above.
Go to : 
Notes
Funding
This study was supported in part by a grant to Jaewon Shim and Jeongtae Kim from Kosin University College of Medicine (2021) and the National Research Foundation of Korea (grant number: NRF-2022R1F1A1074980).
Author contributions
Conceptualization: JS, JK. Investigation: JS, JK. Funding acquisition: JS, JK. Supervision: JS, JK. Writing - original draft: JS, JK. Writing - review & editing: JS, JK. Approval of final manuscript: all authors.
Go to : 
References
1. Russell WMS, Burch RL. The principles of humane experimental technique. London: Methuen;1959.
2. Taylor K, Alvarez LR. An estimate of the number of animals used for scientific purposes worldwide in 2015. Altern Lab Anim. 2019; 47:196–213.

3. Festing S, Wilkinson R. The ethics of animal research: talking point on the use of animals in scientific research. EMBO Rep. 2007; 8:526–30.
4. Smith AJ. Guidelines for planning and conducting high-quality research and testing on animals. Lab Anim Res. 2020; 36:21.

5. Shamoo AE, Resnik DB. Responsible conduct of research. London: Oxford University Press;2009.
6. European Commission. EURL-ECVAM: European Union Reference Laboratory for Alternatives to Animal Testing [Internet]. Brussels: European Commission; c2022 [cited 2022 Dec 7]. https://data.jrc.ec.europa.eu/collection/id-0088.
7. ZEBET. Database for Alternative Methods to Animal Experiments of the Centre for Documentation and Evaluation of Alternatives to Animal Experiments [Internet]. ZEBET; c2000 [cited 2022 Dec 7]. https://apps.bfr.bund.de/animalt-zebet/index.cfm.
8. Edrey YH, Hanes M, Pinto M, Mele J, Buffenstein R. Successful aging and sustained good health in the naked mole rat: a long-lived mammalian model for biogerontology and biomedical research. ILAR J. 2011; 52:41–53.

9. Piper MDW, Partridge L. Drosophila as a model for ageing. Biochim Biophys Acta Mol Basis Dis. 2018; 1864(9 Pt A):2707–17.

10. Linford NJ, Bilgir C, Ro J, Pletcher SD. Measurement of lifespan in Drosophila melanogaster. J Vis Exp. 2013; (71):50068.
11. Munro D, Baldy C, Pamenter ME, Treberg JR. The exceptional longevity of the naked mole-rat may be explained by mitochondrial antioxidant defenses. Aging Cell. 2019; 18:e12916.

12. Shaposhnikov MV, Guvatova ZG, Zemskaya NV, Koval LA, Schegoleva EV, Gorbunova AA, et al. Molecular mechanisms of exceptional lifespan increase of Drosophila melanogaster with different genotypes after combinations of pro-longevity interventions. Commun Biol. 2022; 5:566.

13. Giannakou ME, Goss M, Junger MA, Hafen E, Leevers SJ, Partridge L. Long-lived Drosophila with overexpressed dFOXO in adult fat body. Science. 2004; 305:361.

14. Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol. 2004; 14:885–90.

15. U.S. Food and Drug Administration. Guidance for industry and other stakeholders: toxicological principles for the safety assessment of food ingredients. Redbook 2000. Rockville: U.S. Food and Drug Administration;2007.
16. Han EH, Lim MK, Lee SH, Rahman MM, Lim YH. An oral toxicity test in rats and a genotoxicity study of extracts from the stems of Opuntia ficus-indica var. saboten. BMC Complement Altern Med. 2019; 19:31.

17. Demers G, Griffin G, De Vroey G, Haywood JR, Zurlo J, Bedard M. Animal research: harmonization of animal care and use guidance. Science. 2006; 312:700–1.
18. Leary S, Underwood W, Anthony R, Cartner S, Grandin T, Greenacre C, et al. AVMA guidelines for the euthanasia of animals: 2020 edition [Internet]. Schaumburg: American Veterinary Medical Association; c2020 [cited 2022 Dec 7]. https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download