Introduction
Extraintestinal manifestations (EIM) occur in about 50% of inflammatory bowel disease (IBD) patients over the course of their disease [
1]. IBD consists predominantly of ulcerative colitis (UC) and Crohn’s disease (CD). IBD is a chronic inflammatory gastrointestinal condition and is more commonly regarded as a systemic disease because it is not limited to the gastrointestinal tract. EIMs of IBD may show musculoskeletal, dermatological, vascular, ocular, cardiac, pulmonary, hepatobiliary, genitourinary, hematological, metabolic, or endocrine involvement. However, the incidence of patients with IBD having more than one EIM is rare.
Pyoderma gangrenosum (PG), which is a rare noninfectious neutrophilic dermatosis, infrequently manifests in 1% to 10% of patients with UC; however, because the course of PG is aggressive, it can result in a precarious situation [
2]. The lesion is typically located on the extensor surfaces of the lower extremities, and it may become aggravated when UC is active. However, the correlation between the appearance of skin lesions and clinical activity of intestinal disease is controversial. Venous thromboembolism, occurring in 2.3% to 7% of IBD patients, has a 2-fold higher incidence in patients with IBD than in the general population and can lead to significant morbidity and mortality. In those with an active flare, this risk is 8-fold [
3]. The occurrence of thrombotic complications in IBD is generally attributed to the existing hypercoagulable conditions.
Twenty-five percent of IBD patients have more than one EIM; therefore, a detailed history and physical examination are required to identify EIMs [
4]. Herein, we report a case of multiple EIMs with complicated PG and venous and arterial thromboembolisms in a patient with acute severe UC, successfully treated with biologics. Prior to this report, there were few reports of PG treated with biologics from Asia [
5,
6].
Go to :

Case
Ethical statements: This study was approved by the Institutional Review Board of the Pusan National University Hospital (2204-007-113), and the patient provided signed informed consent.
A 19-year-old female without a medical history presented to the emergency department complaining of bloody diarrhea lasting for 3 months. She complained of hematochezia 10 to 12 times a day and ulcerations on her right foot. She explained painful papules had appeared 2 months ago and rapidly worsened to ulcerations. On physical examination, 3×10-cm oval-shaped ulceration was noted on the dorsal side of her right foot with necrotic and irregular edges and a purulent base (
Fig. 1A). The patient’s vital signs revealed mild tachycardia and tachypnea.
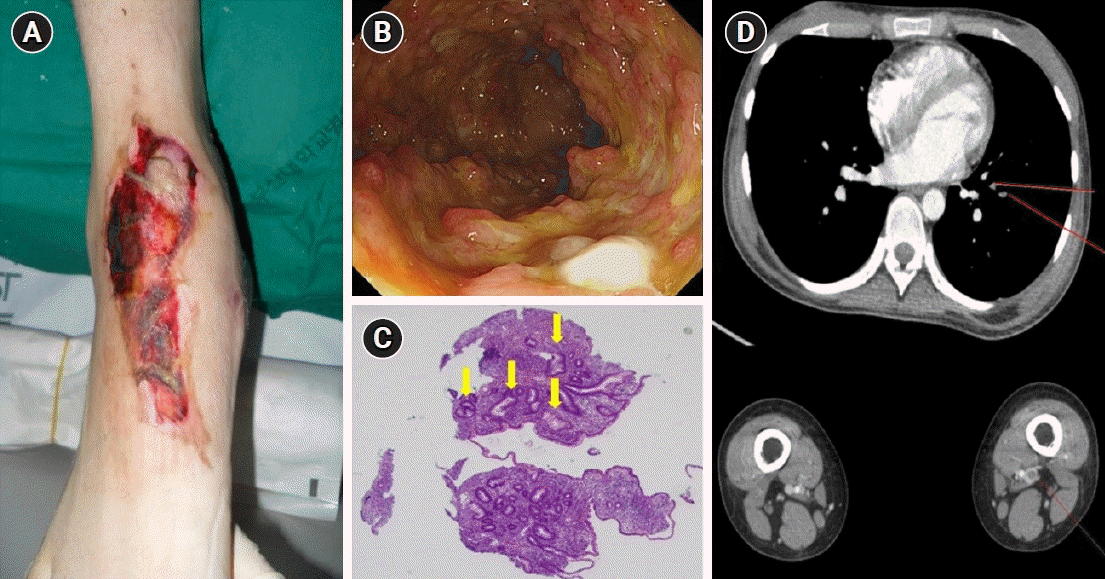 | Fig. 1.Pyoderma gangrenosum and thromboembolism in a patient with acute severe ulcerative colitis before treatment with infliximab. (A) A large skin ulceration with a purulent base and an irregular edge was located on the dorsal side of the patient’s right foot. (B) Colonoscopy revealed diffuse, hyperemic, edematous, colonic mucosa with friability and deep ulcerations. (C) A histopathologic examination of the colonic mucosa showed focal cryptitis and crypt distortion (arrows) with inflammatory cell infiltration (H&E, ×40). (D) Computed tomography revealed pulmonary thromboembolism at the segmental and subsegmental branches of the left lower lobar pulmonary artery and deep vein thrombosis in the left superficial femoral vein. 
|
Laboratory tests revealed a total leukocyte count of 12,500 cells/μL, hemoglobin level of 9.7 g/dL, C-reactive protein of 3.82 mg/dL, erythrocyte sedimentation rate of 65 mm/hr, a total serum protein level of 5.1 g/dL, and a serum albumin level of 2.7 g/dL. Emergency sigmoidoscopy was performed revealing hyperemic and ulcerated colonic mucosa from the rectum to the descending colon. Hyperemic, erythematous, nodular, and ulcerated colonic mucosa with friability was noted from the rectum to the descending colon (
Fig. 1B). Histopathologic examination of the ulcerated colonic mucosa revealed severe, active inflammatory changes and chronic inflammation with cryptitis and crypt distortions (
Fig. 1C). No granulomas or vasculitis were noted. The patient was diagnosed with acute severe UC (Mayo score of 10) based on clinical history, laboratory studies, and biopsies of the colon. The patient was administered intravenous corticosteroids with methylprednisolone given in a dose of 40 mg/day and oral mesalamine, after which her hematochezia partially improved. However, there was no improvement in skin lesion. The dermatologic department was consulted regarding the ulcerated skin lesion, and a histopathologic examination revealed epidermal and superficial dermal necrosis with inflammatory cell infiltration and abscess formation. The patient was diagnosed with PG and administered a topical steroid, antibiotic ointment and hydroactive dressing. She showed improvement of bowel symptoms but no improvement of skin lesions, even after 1 week of intensive steroid therapy. Hence, she was started on intravenous infliximab at a dose of 5 mg/kg after 10 days of steroid use. Her initial Mayo score was 10, but symptoms of UC and PG were gradually improved during hospitalization. Following infliximab administration, the skin lesions showed considerable improvement, evidenced by the reduction in size and the appearance of granulation tissue at the periphery after the second dose of infliximab. Subsequently, she received the remaining one induction dose of infliximab at week 6. At a 6-month follow-up appointment, the patient’s skin lesion was completely healed (
Fig. 2A) and endoscopic findings (Mayo endoscopic score 0) revealed mucosal healing (
Fig. 2B).
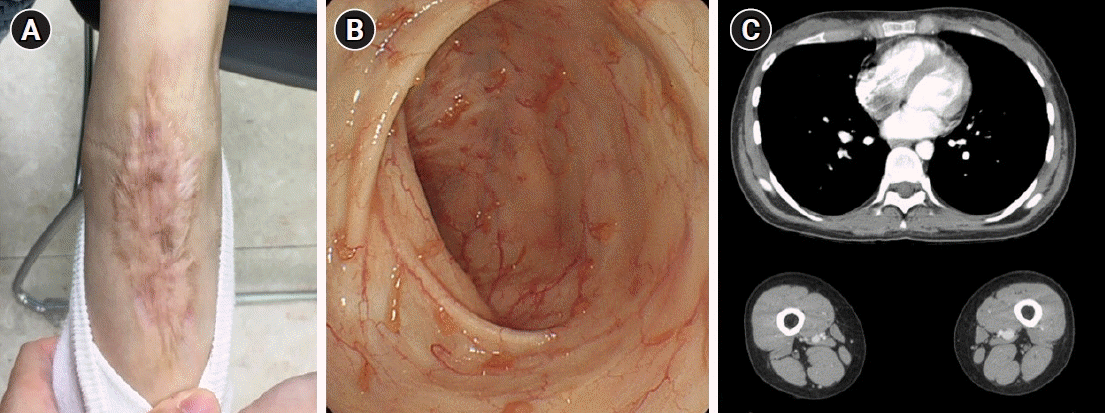 | Fig. 2.Resolution of pyoderma gangrenosum and thromboembolism and endoscopic mucosal healing after treatment with infliximab. (A) The large skin ulceration of the right foot had completely healed at a 6-month follow-up visit. Only a scar remained. (B) Colonoscopy performed 6 months after discharge revealed completely healed colonic mucosa with small inflammatory polyps. (C) Pulmonary thromboembolism and deep venous thrombosis were not detected on a 6-month follow-up computed tomography scan. 
|
She continued to complain of mild dyspnea while in the hospital. A chest radiograph showed no remarkable abnormal findings, and the physical examination and pro-coagulant labs such as protein C, S, anti-phospholipid antibody and antithrombin III level were normal; however, the patient’s D-dimer had increased to 4.72 μg/mL. Computed tomography performed using the thromboembolism protocol revealed a pulmonary thromboembolism located at the segmental and subsegmental branches of the left lower lobar pulmonary artery and a deep venous thrombosis located at the right common iliac vein and left popliteal vein (
Fig. 1D). The patient underwent anticoagulant therapy with low-molecular weight heparin, and her dyspnea gradually improved. Anticoagulation therapy was continued with warfarin, and follow-up imaging after 3 months showed a complete resolution of pulmonary thromboembolism (
Fig. 2C).
Go to :

Discussion
IBD, which includes UC and CD, is a chronic inflammatory disorder of the gastrointestinal tract. However, IBD is more commonly regarded as a systemic disease because it is not limited to the gastrointestinal tract, but it can manifest in any other organs [
7]. EIM are defined as “an inflammatory pathology in a patient with IBD that is located outside the intestine and for which the pathogenesis is either dependent on immune response from the intestine or is an independent inflammatory event” [
8]. EIMs can occur in up to 25%–40% of patients with IBD [
1]. The pathogenesis of EIMs in patients with IBD is poorly elucidated; however, regarded as immune reactions, it is hypothesized that genetically susceptible cells, other than the gut, may be stimulated by an exposure leading to EIMs. Depending on the exposure duration, EIMs can present before the diagnosis of IBD, during the disease course, or even in the remission state or after colectomy.
EIMs are classified into three categories. The first category is colitis related, specifically skin (erythema nodosum [EN] and PG), eye (episcleritis and uveitis), joint (peripheral and axial arthritis), and liver (primary sclerosing cholangitis). These four organs are most frequently affected and regarded as sharing a common immunologic etiology. The second category is EIM secondary to complications due to metabolic or anatomic abnormalities of bowel disease, including thromboembolic events, nephrolithiasis, cholelithiasis, amyloidosis, and pancreatitis. The last category is associated conditions with uncertain mechanism such as insulin-dependent diabetes mellitus, autoimmune thyroid disease, or vitiligo [
9,
10]. Episcleritis, EN, and peripheral arthritis tend to follow the clinical course of IBD; however, uveitis, PG, and primary sclerosing cholangitis may not parallel the course of bowel disease activity [
11].
Cutaneous involvement has been described as prevalent in up to 2%–34% of patients with IBD; EN and PG being the most common cutaneous lesions [
10]. PG is the most serious skin condition, with a prevalence of 1%–10% in patients with IBD, and reported as more common in patients with UC than in those with CD [
2,
7,
12]. The pathogenesis of PG is poorly understood; however, cross antigenicity between the skin and the intestinal mucosal are thought to be responsible for these reactions. Similar to EIMs, PG can present before the diagnosis, during the disease course, or even in the remission state or after colectomy [
7,
13]. PG usually begins as a small erythematous, indurated skin lesion which evolves rapidly to an enlarged necrotic ulcer with irregular violaceous edges. PG can occur on other sites of the body including hands, back, neck, and breasts; however, lesions are commonly found on the extensor surfaces of the lower limbs [
13]. Histological characteristics include perivascular lymphocytic infiltrate with prominent dermal neutrophilic infiltration, and a central abscess [
11]. The management of PG is divided into two categories: topical and systemic treatment. Topical treatment includes local wound care and local pharmacologic treatment such as intralesional corticosteroid and topical 5-aminosalicylic acid. Systemic treatment includes corticosteroids, azathioprines, 6-mercaptopurines, and biologics. Mild cases can be treated with topical therapy; however, severe cases may be treated in conjunction with systemic treatment. Tumor necrosis factor-α inhibitors are considered as first-line drugs in management of refractory disease and should be administered promptly in the case of delayed response to corticosteroid therapy [
7,
11]. The data demonstrating outcomes of management of UC-related PG with biologics are still limited, but there is strong evidence for the use of biological therapy. PGs with biologics are still limited, and there is compelling evidence for the use of biologic agents. Although both infliximab and adalimumab have been successfully used to manage UC-related PGs, infliximab is more effective and is the treatment of choice. In this case, infliximab was administered because a rapid response to corticosteroids was not achieved in the management of PG. In our report, patients showed successful outcomes. Complete healing of skin wounds (with scarring) was documented at 6 months after the start of biologic therapy.
Thromboembolic events are also serious EIMs complicating the course of IBD and can lead to substantial morbidity and mortality [
14]. Patients with IBD have an increased risk of vascular complications, approximately 2- to 3-fold higher compared with the non-IBD population [
15]. The prevalence of thromboembolic events among patients with IBD is up to 7% [
16]. Thromboembolic events usually present as deep vein thromboses in the legs or as pulmonary emboli, are more common in younger compared to older individuals, and recur frequently. Known risk factors are active or complicated disease, extensive colonic involvement, recent hospitalization, and surgery [
17,
18]. The pathogenesis of thromboembolic events is unknown; however, multiple acquired factors, such as venous stasis, vascular endothelial injury, and hypercoagulable state and inherited factors, are thought to be involved [
14]. Management of thromboembolic events is composed of primary prevention and treatment with secondary prevention. Increased risk of thromboembolic events is thought to be a suitable indication for primary prophylaxis in IBD patients [
19]. In the case of obvious thromboembolism, patients are treated with anticoagulation and in severe cases, thrombolysis is often required. Low-molecular weighted heparin is most often used for primary prevention and treatment of thromboembolism, and the duration varies from 3 months to lifelong, depending on the individual cases [
14,
19]. In our present case, the patient was treated with anticoagulation with low-molecular weighted heparin and was continued with warfarin. The deep vein thrombosis and pulmonary embolism was resolved completely after 6 months.
Approximately one-quarter of the patients with IBD present with multiple EIMs [
6]. The development of one EIM seems to increase the risk of developing other EIM, because it is hypothesized that joint, skin, eye, and biliary tract share a common immunologic pathway [
10]. In previous reports, risk factors for multiple EIMs in patients with IBD were a colonic disease, active disease, and positive family history [
10,
20]. In our case, the patient had active colonic disease; therefore, the risk of multiple EIMs might have been higher.
In conclusion, the patient in this case report could be diagnosed as UC with PG and pulmonary embolism, due to careful history taking and physical examination. She received infliximab for the management of UC and PG and anticoagulation medication for deep vein thrombosis and pulmonary embolism; these were resolved without any complications. EIMs are thought to share a common immunologic pathway and various extrinsic gastrointestinal symptoms can be presented in patients with IBD. Therefore, physicians should improve their awareness of the prevalence, associated risk factors, and the management of EIMs. Furthermore, thorough consideration and attention should be paid and adequate evaluation should be performed in the management of patients with IBD presenting EIMs.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download