Abstract
Anhydrous ammonia is a commonly used chemical in industry. Ammonia gas inhalation causes thermal injuries and alkali burns in the airway and lung parenchyma. Previous case reports have stated that respiratory sequelae after acute ammonia inhalation burns were associated with structural lung disease, such as bronchiectasis or interstitial lung disease. We herein report two cases of long-term sequelae with persistent airflow limitation after ammonia inhalation burns.
Anhydrous ammonia (NH3) is a colorless gas with a pungent odor at room temperature. It is a commonly used chemical in the industry for refrigeration and in agriculture as a fertilizer. It is a highly hydrophilic gas and when it is dissolved in water it forms ammonium hydroxide (NH4OH) which is a strong alkali substance. When a human is exposed to ammonia gas, it dissolves to water-rich body parts, such as the eyes, skin, gastrointestinal tract, and airway mucosa. This is an exothermic reaction and causes thermal injury and alkali burn to the target organ [1].
Acute exposure to ammonia gas causes various respiratory diseases, such as laryngitis, bronchitis, bronchopneumonia, and pulmonary edema [2]. In severe cases, patients die due to respiratory failure [3]. Even after recovering from an acute lung injury, victims experience chronic respiratory diseases, such as bronchiectasis [4], obstructive bronchiolitis [5], interstitial lung disease [6], chronic obstructive lung disease [7], and end-stage lung disease requiring lung transplantation [8]. Several of these reported cases were accompanied by structural changes in the lung parenchyma and bronchi.
We experienced two cases of ammonia inhalation injury who complained of persistent wheezing, dyspnea, cough, and sputum. In both cases, patients did not have the above-mentioned symptoms especially asthma or allergic diseases before the accidental exposure to ammonia and had no smoking history. Herein, we report obstructive lung disease that developed after ammonia gas exposure.
Ethical statements: This study was approved by the Ethics Committee of Dong-A University Hospital (No. DAUHIRB-21-212). The need for written informed consent from the participants was waived because of the retrospective nature of this study.
Twenty people were exposed to ammonia gas due to damage to a refrigeration system while repairing a fish tanker at a local shipyard. One of them died and 19 were injured, and they were treated at a local emergency center after the accident. Two of the 19 injured patients visited our hospital after the accident due to persistent dyspnea, which was limiting their daily life.
A previously healthy and non-smoker, a 61-year-old male experienced burns on approximately 10% of his total body surface area (upper back, head, right leg, scrotum, and left cornea) after exposure to ammonia gas and was treated in an intensive care unit with mechanical ventilation due to accompanying respiratory distress. The initial computed tomography (CT) scan of the lung showed atelectasis (Fig. 1A) and bilateral multiple patchy consolidations (Fig. 1B). Extubation was performed 14 days after the accident, the burn treatment was continued, and the patient was discharged 40 days after the accident; however, wheezing, productive cough, and dyspnea were maintained after the accident. In the lung function test, the forced expiratory volume in 1 second (FEV1)/the forced vital capacity (FVC) was 57%, FEV1 was 40% and FVC was 69% of the predicted values (Fig 2A). And in the bronchodilator test, FEV1 and FVC changes were checked as 2% and 0%, respectively, therefore no airway reversibility was observed.
He was referred to our hospital for sustained respiratory symptoms despite being treated with a bronchodilator and systemic corticosteroid. He complained of modified Medical Research Council grade 2 dyspnea, cough, sputum, and wheezing. A CT scan taken 6 months after the accident revealed focal tubular bronchiectasis with mucus impaction in the right middle lobe and both lower lobes (Fig. 1C). Bronchoscopy was performed and some scars appeared as burn wounds in the left upper lobe area with no other specific findings (Fig. 3A). In the follow-up pulmonary function test (PFT), obstructive ventilatory defect persisted and there was no significant difference from the previous results (Fig. 2A).
During the 6-year follow-up period, despite continuous medical treatment with mucolytics, bronchodilators and inhaled corticosteroid, he was hospitalized 11 times due to worsening of the symptoms (exacerbation of dyspnea, wheezing, and sputum). In the follow-up CT scan (Fig. 1D) and bronchoscopy (Fig. 3B), there was no structural change compared to the previous results, and airflow limitation persisted in lung function tests.
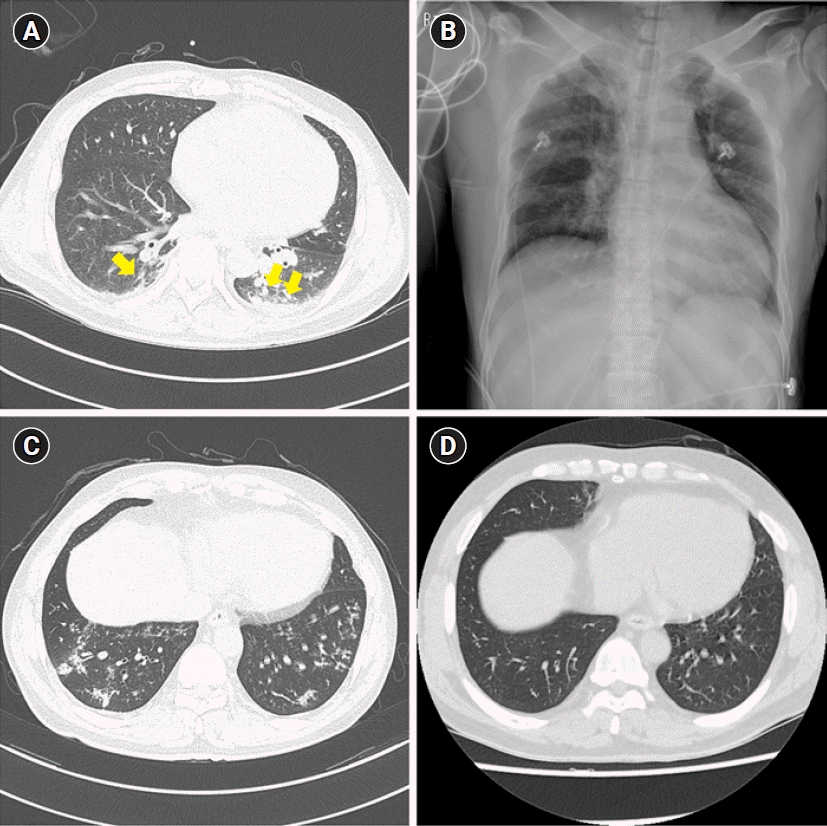
A previously healthy and non-smoker, a 51-year-old male was transferred to the emergency department of our hospital with tracheal intubation after an ammonia exposure accident. He experienced rhabdomyolysis and burns on approximately 1% of the total body surface area (scrotum and both cornea). At the initial chest CT scan, ground-glass opacity pattern pneumonitis was found in both lower lobes (Fig. 4A). Extubation was performed 7 days after the accident and the patient was discharged 14 days after the accident. One month after the accident, the patient visited the outpatient clinic with modified Medical Research Council grade 2 dyspnea, sputum, cough, and wheeze. He had no allergic disease history like allergic rhinitis or asthma. Initial chest X-ray (Fig. 4B) showed no definite lung lesion.
Five months later, he consistently complained of dyspnea, cough, and wheezing. An initial PFT was performed after 6 months after the accident and the result was the FEV1/FVC 65%, FEV1 68%, and FVC 81% of the predicted values (Fig. 2B). And in the bronchodilator test, FEV1 and FVC changes were checked as 3% and 0%, respectively, therefore no airway reversibility was observed. The chest CT scan after 12 months showed newly developed multifocal ground-glass opacity with consolidation; however, the initial lesion showing ground-glass opacity disappeared (Fig. 4C). Flexible bronchoscopy was performed and no endobronchial lesions were found (Fig. 3C).
The patient is under follow-up for over 6 years so far. He still experiences from dyspnea and wheezing. A follow-up chest CT scan after 24 months revealed an improved state of the previous lesion and there were no structural changes (Fig. 4D). Also, no endobronchial lesions were found at follow-up bronchoscopy (Fig. 3D). Follow-up PFTs revealed fluctuating changes in the FEV1 (62%–82% of the predicted value); however, there was a persistent airflow limitation (Fig. 2B).
Airway damage due to ammonia inhalation burn is caused by the following mechanism. First, tracheal epithelial cells are directly damaged by thermal injury. After the inhalation of anhydrous ammonia gas, it dissolves into the airway lining fluid forming ammonium hydroxide. As mentioned before, this is a highly exothermic reaction [1]. The heat produced from this reaction, causes protein degeneration of the airway lining epithelial cells, which leads to the desquamation of the bronchial epithelium and necrosis [9]. Second, the reaction produces a strong alkaline solution, causing liquefaction necrosis and worsening airway damages [1]. Third, thermal and alkaline injuries cause the secretion of pro-inflammatory cytokines. These cytokines trigger airway edema and the activation of protease, accelerating airway damage [9].
Although the development of chronic obstructive lung disease following smoke inhalation injury is rare, there is some evidence that prolonged airway hyper-responsiveness and asthma-like symptoms may follow smoke inhalation [7]. Cha et al. [10] reported isolated thermal injuries from smoke inhalation in a subway fire in Daegu, South Korea. In this study, victims experienced dyspnea, cough, and wheezing, and they revealed a reduced FVC and FEV1 in the initial PFT. However, after 3 months of follow-up, the results of the PFT recovered to the normal range which was maintained for 6 months. Although, the follow-up period was relatively short, the authors concluded that this phenomenon may be the result of certain physiologic effects, such as bronchospasm. Whitener et al. [11] and Fogarty et al. [12] also reported on the relationship between thermal inhalation injury and PFT. In these studies, the initial PFT showed obstructive airflow limitation, and it was maintained during long-term follow-up periods, such as 5 months and 2 years each without structural lung injury.
On the contrary, there are reports that chronic occupational exposure to ammonia and wood smoke and firefighters are associated with chronic airflow limitation [13]. Ballal et al. [14] reported that chronic exposure to a high concentration of ammonia could cause bronchial asthma in healthy people who resided near two fertilizer-producing factories. Other studies reported that continuous occupational exposure to ammonia was associated with the exacerbation of asthma [15]. However, the pathogenesis between asthma onset and ammonia exposure was not explained in those reports.
In previous cases with airflow limitation after acute ammonia inhalation, the cause of airflow obstruction was associated with structural lung diseases, such as bronchiectasis or lung fibrosis [4]. In case 1, focal bronchiectasis was found on chest CT. However, the confined lesion was not enough to explain moderate airflow obstruction and wheezing sound in both whole lung fields. In case 2, chest CT and bronchoscopy showed no parenchymal or airway damage. To the best of our knowledge, this is the first report about newly developed asthma-like chronic obstructive lung disease proven by 6 years of PFT results after short-term exposure to massive ammonia inhalation.
The pathogenesis of development of asthma-like obstructive lung disease after acute ammonia exposure is unknown. Previously reported ammonia inhalation burn cases were associated with structural lung disease, suggesting that the main damage site is the large airway. In our case, damage caused by ammonia seems to have occurred mainly in the small airways, which may be related with massive amount of exposure and subsequent small airway inflammatory reaction might lead to the development of obstructive lung disease. However, further studies about pathogenesis are needed in the future.
In conclusion, we have examined two patients who developed asthma-like respiratory symptoms with persistent airflow limitation after accidental ammonia gas exposure.
Notes
Author contributions
Conceptualization: SJU. Data curation: HL, YHN. Formal analysis: BHK, DHL. Investigation: YHN, MSR. Methodology: BHK, DHL, MSR. Project administration: SJU. Resources: HL, BHK. Supervision: MSR, SJU. Validation: IK, SJU. Visualization: BHK, DHL, MSR. Writing - original draft: IK, IH. Writing - review & editing: IK, SJU. Approval of final manuscript: all authors.
References
2. Tonelli AR, Pham A. Bronchiectasis, a long-term sequela of ammonia inhalation: a case report and review of the literature. Burns. 2009; 35:451–3.

3. Zhang F, Zheng XF, Ma B, Fan XM, Wang GY, Xia ZF. Mass chemical casualties: treatment of 41 patients with burns by anhydrous ammonia. Burns. 2015; 41:1360–7.

4. Leduc D, Gris P, Lheureux P, Gevenois PA, De Vuyst P, Yernault JC. Acute and long term respiratory damage following inhalation of ammonia. Thorax. 1992; 47:755–7.

6. Brautbar N, Wu MP, Richter ED. Chronic ammonia inhalation and interstitial pulmonary fibrosis: a case report and review of the literature. Arch Environ Health. 2003; 58:592–6.

7. de la Hoz RE, Schlueter DP, Rom WN. Chronic lung disease secondary to ammonia inhalation injury: a report on three cases. Am J Ind Med. 1996; 29:209–14.

8. Pirjavec A, Kovic I, Lulic I, Zupan Z. Massive anhydrous ammonia injury leading to lung transplantation. J Trauma. 2009; 67:E93–7.

9. Jones SW, Williams FN, Cairns BA, Cartotto R. Inhalation injury: pathophysiology, diagnosis, and treatment. Clin Plast Surg. 2017; 44:505–11.
10. Cha SI, Kim CH, Lee JH, Park JY, Jung TH, Choi WI, et al. Isolated smoke inhalation injuries: acute respiratory dysfunction, clinical outcomes, and short-term evolution of pulmonary functions with the effects of steroids. Burns. 2007; 33:200–8.

11. Whitener DR, Whitener LM, Robertson KJ, Baxter CR, Pierce AK. Pulmonary function measurements in patients with thermal injury and smoke inhalation. Am Rev Respir Dis. 1980; 122:731–9.

12. Fogarty PW, George PJ, Solomon M, Spiro SG, Armstrong RF. Long term effects of smoke inhalation in survivors of the King’s Cross underground station fire. Thorax. 1991; 46:914–8.

13. Moran-Mendoza O, Perez-Padilla JR, Salazar-Flores M, Vazquez-Alfaro F. Wood smoke-associated lung disease: a clinical, functional, radiological and pathological description. Int J Tuberc Lung Dis. 2008; 12:1092–8.
14. Ballal SG, Ali BA, Albar AA, Ahmed HO, al-Hasan AY. Bronchial asthma in two chemical fertilizer producing factories in eastern Saudi Arabia. Int J Tuberc Lung Dis. 1998; 2:330–5.
Fig. 1.
The chest computed tomography (CT) scan of case 1. (A) The initial chest CT scan with atelectasis (arrows) and (B) patch
consolidation (arrows). The follow-up chest CT scan after 6 months with (C) atelectasis (arrowheads) and tubular bronchiectasis with mucus impaction (arrows) (D) after 1 year.
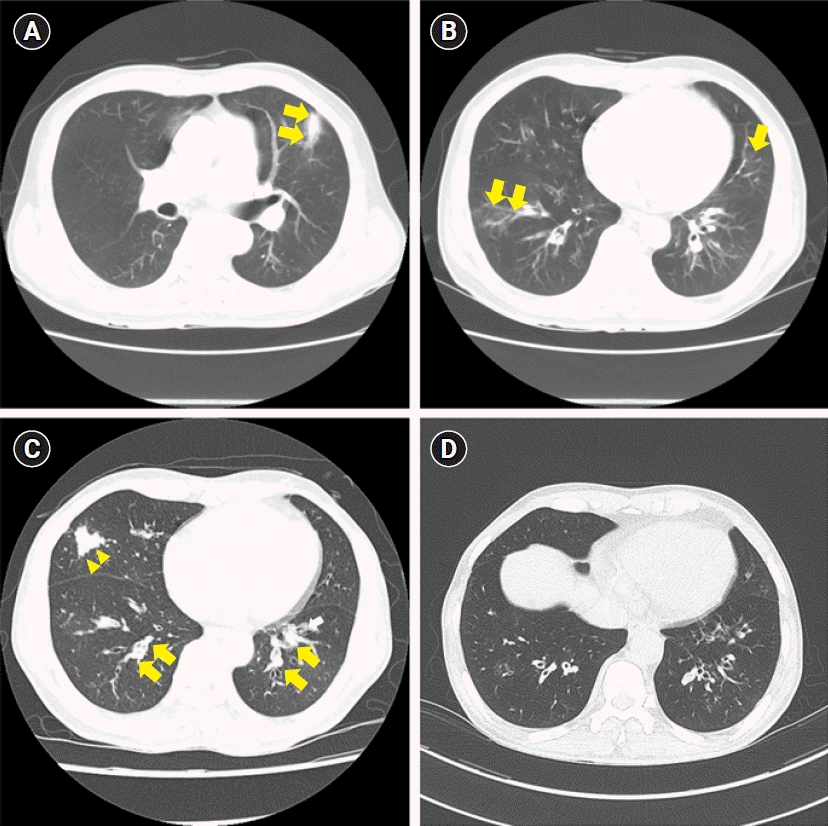
Fig. 2.
Serial pulmonary function test results of cases 1 (A) and 2 (B). FVC, forced vital capacity; FEV1, forced expiratory volume in 1 second; Ref, reference.
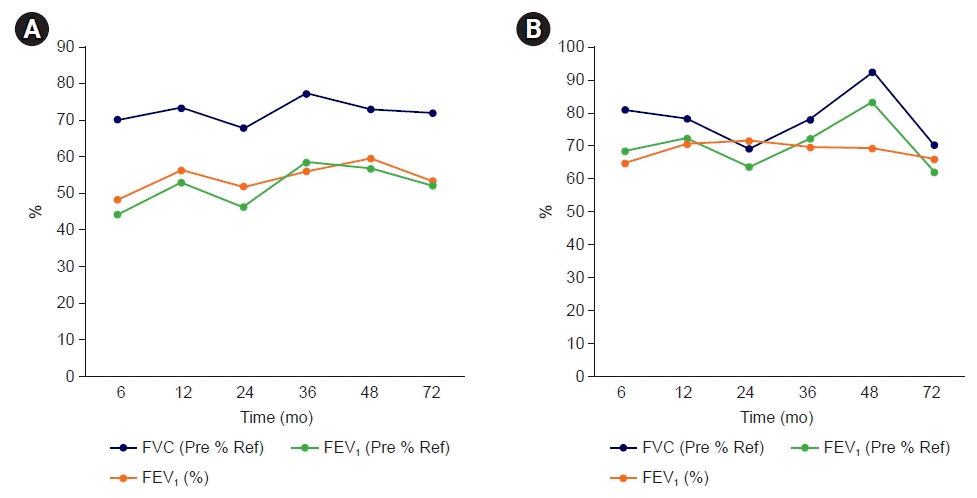
Fig. 3.
Bronchoscopy findings of cases 1 and 2. Chemical burn scar changes (arrows) on bronchoscopy performed at 6 months (A) and at 36 months (B) after exposure in case 1. Bronchoscopy findings at 6 months (C) and at 36 months (D) after exposure in case 2.
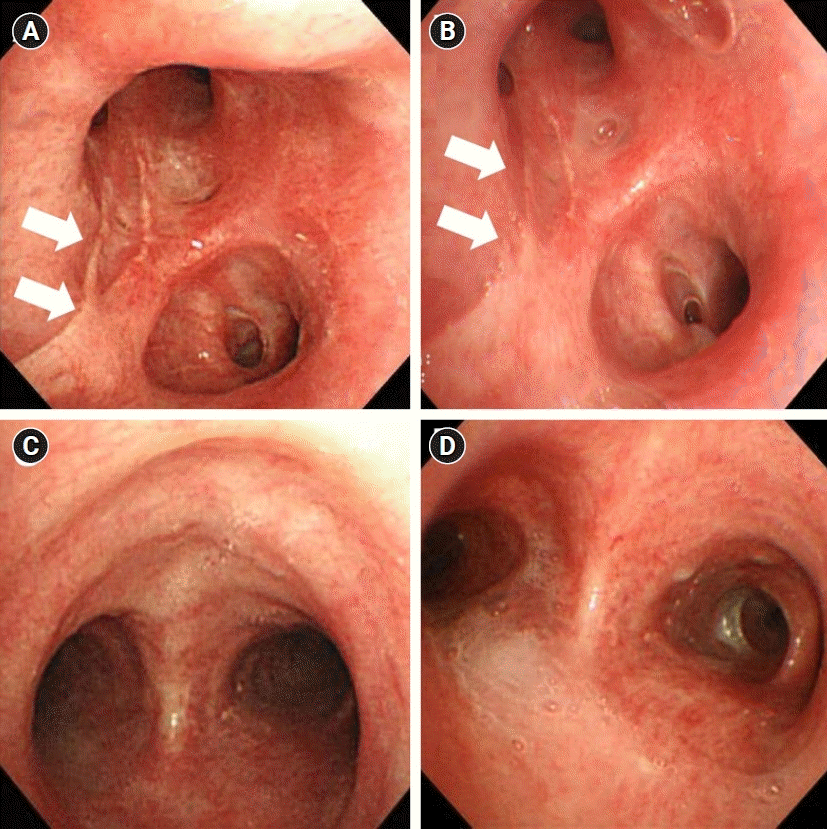
Fig. 4.
Chest computed tomography (CT) scans and chest X-ray image of case 2. (A) The initial chest CT scan with diffuse ground-glass opacities (arrows) in both lower lobes. (B) The initial chest X-ray. (C) A follow-up chest CT scan after 12 months with multifocal ground-glass opacities and consolidations. (D) A follow-up chest CT scan after 24 months with no other abnormalities.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download