Abstract
Objective
Numerous classification schemes have been used for carotid cavernous fistula (CCF), each describing some aspect of the disease process but none of them provides a complete description of the fistula including its clinical features, natural history, arterial and venous architecture.
Methods
Retrospective clinical and radiological review was done for all the patients diagnosed with CCF and treated at our institute. The CCF were classified according to the proposed API-ACE classification along with Barrow and Thomas classification.
Results
Overall 28 patients (M=21, F=7) were diagnosed and treated during the 6-year period. 89.2% of CCF developed following an episode of head injury. Orbital symptoms were the most common presenting complaints. Barrows type A was the most predominant subtype (n=24) and most of the patients (n=23) demonstrated decreased ipsilateral carotid filling. Combined anterior and posterior drainage pattern was the most common drainage pattern and anterior drainage was more commonly observed than posterior drainage.
Carotid cavernous fistula (CCF) is defined as abnormal communication, either direct shunt between internal carotid artery (ICA) and veins in the region of cavernous sinus (CS) or indirect shunt between branches of ICA or external carotid artery (ECA) and veins in this region including dural arteriovenous fistulas (DAVFs) [14]. Historically, numerous classification systems have been used to categorize CCF based on: aetiology (traumatic or spontaneous), hemodynamic (high flow vs low flow) or angiographic characteristics (direct vs indirect). However, the classification system proposed by Barrows (BC) et al. was the first detailed scheme based on the arterial angioarchitecture of the lesion [3]. The major criticism of this classification was based on the fact that it didn’t incorporate the flow characteristics of the lesion consequently leading to its inability to predict clinical presentation and hemorrhage risk. Various other schemes were proposed to overcome this shortcoming like Borden and Cognard classification but they were limited to DAVFs and couldn’t differentiate between the predominant direction of venous drainage in CCF [4,6]. Thomas et al. recently published a new classification system using venous drainage which could guide treatment approach while also correlating with clinical presentation and outcome [13]. We present our experience in the endovascular treatment of CCF and attempt at better delineation of their arterial and venous angioarchitecture.
A retrospective review was carried out of all diagnosed CCF cases presenting to our institution who underwent endovascular treatment between the period of march 2013 to February 2019 after obtaining clearance from institutional ethical committee. The cases were selected after reviewing the records in the endovascular suite and the pertinent inpatient, operative, angiographic and follow up records were retrieved and analysed. Demographic and clinical data were assessed and recorded. Diagnostic cerebral angiography images were reviewed for all patients delineating arterial and venous architecture as well as characteristics of the fistula. The review of pre-operative images, decision making and endovascular intervention was done by two independent neuro-interventionalists and any discrepancy was resolved by mutual consensus.
According to the Barrow classification (BC), CCF were categorized as type A (direct shunt between ICA and CS), type B (shunt between ICA branch and CS), type C (CCF fed by ECA branches), type D (CCF involving branches of both ICA and ECA) [3]. The cases were further evaluated to determine the location and size of fistulous communication along with distal ICA flow [7]. Filling of fistula from either contralateral ICA or posterior communicating arteries (PCOM) was also reviewed.
CCF were classified according to their venous anatomy using Thomas classification (TC) as type 1 (drainage present posteriorly: inferior and/or superior petrosal sinuses and/or inferiorly: pterygoid and parapharyngeal plexus), type 2 (posterior/inferior and anterior: superior and inferior ophthalmic veins drainage), type 3 (anterior drainage only), type 4 (retrograde drainage into cortical veins ± other routes of venous drainage), type 5 (highflow direct shunt between cavernous internal carotid artery and cavernous sinus (Barrow type A) ± multiple routes of venous drainage) [13]. The cases were further classified according to our proposed classification system to further define the arterial and venous anatomy (Table 1).
Angiogram in a post-traumatic CCF cases showing communication of cavernous sinus with ICA is described in Fig. 1. A modified classification system to better describe the arterial as well as venous anatomy of carotidcavernous fistula is API-ACE classification (Fig. 2). API-ACE can serve as a useful tool to summarize arterial and venous angioarchitecture of direct as well as indirect CCF which is easy to use and enables planning of the endovascular intervention.
Location of CCF is divided into Zone 1, 2, 3 from proximal to distal based on cavernous segment of ICA.
The endovascular intervention was done using either arterial (via ICA) or venous routes (anteriorly through cavernous sinus into superior ophthalmic vein or posteriorly via inferior petrosal sinus). The occlusion of fistula was achieved using coiling or embolization using balloons or a combination of these modalities. Vessel sacrifice was done in few chosen fistulas which showed good collateral circulation and couldn’t be occluded by the aforementioned techniques. Post procedural angiography was done in all the cases to check for the completeness of fistula occlusion and consequent changes in the hemodynamic.
Patients were followed up clinically for resolution of symptoms like visual and ocular complaints and radiological evaluation was done if symptoms progressed or new symptoms emerged. Repeat evaluation and endovascular intervention was planned for cases with clinical and radiological recurrence. The duration of follow up ranged from 14 to 64 months.
Our study included a total of 28 patients with majority being males (75%) with age ranging from 14 to 83 years with median age of 32 years. Two female patients were concomitantly diagnosed with Ehler-Danlos Syndrome and vascular dysplasia respectively. One female patient was found to harbour simultaneous bilateral unruptured internal carotid artery aneurysms but the phenotype did not fit into any recognized syndrome.
89.28% of our study population was found to develop CCF following a traumatic episode (n=25) with only three cases having a non-traumatic spontaneous onset. Ocular symptomology (vision loss, proptosis and chemosis) was the most common presenting complaints in our series (Table 2). No patient was found to suffer from intracranial hemorrhage or seizures. Six patients were in the state of impaired consciousness at the time of presentation.
The angiographic arterial anatomy is summarized in Table 2. Barrows type A was the predominant fistula subtype in our study accounting for 85.71% (24/28) out of which three had a non-traumatic etiology. Three fistulas couldn’t classically be categorized in the Barrows classification as they had direct high flow communication between cavernous sinus and ICA along with contributions from external carotid branches (Fig. 1A). These three patients were classified as type D fistula for descriptive purposes. There was no type B or type C fistula in our study population.
Zone 1 was the most common site of fistula (n=18) with most of them being Barrows type A (n=15). One patient had two communications between ICA and cavernous sinus, at proximal genu and horizontal cavernous segment (Fig. 1B). 14 out of 28 fistulas were large fistula (Table 3) with sumping from either PCOM (n=6) or contralateral ICA (n=2) or both (n=6). Twenty-three patients demonstrated decreased ipsilateral carotid filling with eight of them having no flow.
Anterior outflow (n=26) via superior and inferior ophthalmic veins was the most common venous drainage pattern followed by posterior drainage (n=23) (Table 4). Contralateral cavernous sinus filling was seen in 10 cases and cortical venous efflux in 16 patients of which three also had posterior fossa venous efflux. Using Thomas system for classification, type 5 fistula (n=24) was found to be the most frequent occurrence as most of the fistulas in our series were Barrows type A (Table 5). Even though 10 cases had cortical venous reflux which is included in Thomas type 4, eight of them were classified in type 5 as they had direct, high flow communication with ICA.
Using the API-ACE classification, 27 cases were classified as having direct high flow communication with ICA (A1), three out of which were concurrently having connections with ECA (E1) (Fig. 3). There was only one case with A0E1 designation. AP±I – A1C1E0 (n=13) was the most common type of angio-architecture followed by AP±I – A1C0E0 (n=5) (Fig. 4). Anterior drainage alone was present in 4 cases whereas posterior drainage alone was seen only in two cases (Fig. 5).
All patients underwent endovascular intervention with average time interval from presentation to treatment being 7-14 days. Overall 34 procedures were performed on the 28 included cases with 4 patients needing multiple procedures. Two patients required three procedures each and two needed two sittings for successful control of fistula. Coil embolization of the fistula was the most common procedure performed followed by balloon occlusion (Table 6). Thirty-two procedures were performed via transfemoral trans-arterial approach and two via transfemoral transvenous approach (both via inferior petrosal sinus route).
The post procedure angiography showed complete obliteration of the fistula in 18 out of 28 cases and partial closure with improvement in the flow dynamics was seen in 10 patients. However, on follow up 22 patients experienced complete symptomatic resolution and 5 patient had partial resolution of their presenting symptoms (proptosis, chemosis and orbital bruit). One patient experienced clinical and radiological recurrence following complete occlusion on post procedure angiography due to opening up of other supplying channels. There was one death in the series from an unknown acute event at 10-months post-procedure.
An abnormal communication between ICA or its branches and cavernous sinus results in the transmission of high-pressure arterial blood into CS and its draining tributaries, consequently leading to venous hypertension. The clinical manifestations of CCF is therefore due to increased intracavernous pressure with consequent changes in the venous and arterial flow patterns. Normally the CS receives venous drainage anteriorly from superior and inferior ophthalmic veins and laterally through sphenoparietal sinus, sylvian veins, and cortical veins which ultimately drains posteriorly into jugular bulb through superior/inferior petrosal sinus, inferiorly into pterygoid and para-pharyngeal plexus through various emissary veins and contralaterally through opposite CS [7,12]. However, intracavernous hypertension leads to revised venous drainage which can be anteriorly towards ophthalmic veins, posteriorly or inferiorly towards petrosal sinus or pterygoid/parapharyngeal plexus or laterally in the spheno-parietal sinus or middle cortical veins but most commonly it is a multidirectional drainage. The natural history, clinical manifestations as well as associated complications are direct consequence of the altered hemodynamics and flow pattern.
The most commonly used Barrow Classification describes only the arterial anatomy of the lesion i.e. direct or indirect fistulous connection and consequent high or low flow lesions [3]. There was therefore, need for a system which classifies CCF according to venous architecture which could better predict the natural history, clinical presentation as well as guide the endovascular approach to their treatment. Thomas et al. recently devised a classification scheme centered on similar principles [13].
Three-fourth of our study population consisted of young male patients who developed CCF following head trauma (traumatic CCF 89.2%). Other studies have also reported a high incidence of traumatic CCF with male predominance but the proportion of traumatic CCF varied from 31% in the study by Thomas et al. to 66% by Malan and colleagues [11,13]. Being a developing country with increasing number of automobiles may be responsible for higher number of road traffic accidents with consequent higher number of traumatic fistulas. Ocular symptomatology resulting from anterior venous reflux was the predominant mode of presentation in our series which is similar to that reported by other studies [8,10]. Six out of 28 patients were unconscious at presentation but it might be attributed to the preceding history of head injury which was present in all the cases suffering from altered sensorium rather than a manifestation of CCF. Consciousness improved in all these cases following treatment of CCF over a range of one to 12 weeks. Another remarkable finding was complete absence of cortical symptoms, i.e. seizures or spontaneous intracerebral hemorrhage (ICH) secondary to cortical venous efflux despite the presence of angiographic cortical venous efflux in 57.1% of cases. Thomas et al. reported cortical symptoms in 66% patients and Leone observed cortical symptoms in 50% of their patients with demonstrable cortical efflux [10,13]. The lower observed incidence of cortical symptoms may be attributed to early diagnosis and surgical intervention (within a week of diagnosis) as most of the patients were diagnosed with CCF during the initial period of hospitalization following head injury therefore intervening at an early stage in the natural history of the disease.
Barrow type A fistula was found to be the most common type (85.71%) which can be expected owing to the traumatic aetiology in majority of our patients (89.2%). The incidence of Barrow type A fistula reported in different studies varies from 16.7% (Alam et al.) [2], 24.1% (Thomas et al.) to 26.5% (Leone et al.) with majority of studies showing type D fistula to be the most common type [10,13]. We observed only four type D fistula in our study. Zone 1 was seen to be the most common site of fistula similar to that reported in other studies probably because the carotid artery is more fixed in the region [7,13]. Fifty percent of fistulas in our study were large in size which is similar to that reported by Malan et al. but the fistula size have not been uniformly commented upon in many of the studies [11]. Large fistulas can produce deficits secondary to decreased flow in ipsilateral ICA or sumping of blood flow from PCOM or opposite ICA.
According to the Thomas classification, type 5 fistulas (n=24) were the most common subtype followed by two cases each of type 2 (n=2) and type 4. However, looking at the venous architecture, anterior drainage was the most common pattern (n=26) followed by posterior drainage (n=23). Cortical venous reflux was seen in 16 out of 28 patients with posterior fossa reflux seen in three patients. Giuseppe and colleagues, similarly reporting on their experience of using the Thomas classification found type 3 CCF to be the most common followed by type 5 (n=25) and the classification was seen to correlate significantly with the symptoms as well as the treatment approach [10].
However, in our experience there seems to be an overlap in the classification system, principally due to the definition adopted for type 5 fistula (Barrow type A fistula with or without multiple routes of venous drainage). In our series as the majority of cases were Barrow type A (n=24), they were categorized as TC type 5 even if they were not associated with multiple routes of venous drainage (Table 7). This resulted in diminished discriminating power of the Thomas classification in delineating the venous anatomy as well as suggesting the endovascular assess routes possible. It is evident from the data that type A CCF can also have varied anatomy with different patterns of venous drainage. API-ACE classification describes the arterial as well as the venous architecture of carotid-cavernous-fistula. It also removes the inability of Barrow classification to classify few rare CCF cases with direct ICA along with indirect ECA connections which were encountered in our series (n=3) [1]. Using the API-ACE classification, the venous anatomy including the presence of cortical efflux is well delineated inspite of the arterial supply. This classification also helps in depicting the trans-venous approaches available for endovascular occlusion of the fistula besides describing the natural history and the clinical presentation.
Overall 32 out of 34 procedures were carried out via trans-arterial approach and only two via trans-venous route which is understandable as majority were direct fistulas. This finding is in accordance with those reported by Chi et al. in their series of 172 direct traumatic CCF patients where they utilized trans-venous approach in only one case [5]. Coil was the most commonly employed material for embolization of fistula followed by detachable balloon. The post procedure angiography showed complete resolution of fistula in 64.28% of patients with partial resolution seen in rest of the patients. This is in contrast to the fistula closure rates reported by other studies which falls in range of 84-89% [9,10]. However the clinical resolution was seen in 78.5% of patients which is better than that achieved by Jung et al. and similar to that reported by Leone [10]. Decreased rates of complete occlusion could be due to higher proportion of large fistulas with complex anatomy and multiple feeding vessels as well as due to economic constrains in deciding the modality and means of treatment, ours being a developing country.
The potential limitations associated with this study is its retrospective nature and the inherent bias associated with it as well as the smaller sample size. Another shortcoming was relatively skewed study population, more heavily inclined towards traumatic direct fistulas with relative sparsity of indirect type which could be due to high-level tertiary trauma center associated with the institution. The treatment protocols could be influenced by the economic condition and affordability of the patients. The proposed classification system still doesn’t account for the size of fistula, bilateral CCF cases or those fistula with connection to opposite cavernous sinus.
CCF is a complex disease with varied clinical manifestations dictated by their arterial and venous anatomy. Using a novel classification scheme, we present an attempt to better understand and classify the angioarchitecture of CCF which could help better understand the clinical manifestations and guide in appropriate endovascular approach selection for treatment.
REFERENCES
1. Abecassis IJ, Morton RP, Kim LJ, Ghodke BV, Levitt MR. Combined direct and indirect traumatic carotid-cavernous fistula (CCF): case report and review of the literature. J Clin Neurosci. 2017; Oct. 44:240–42.

2. Alam MS, Jain M, Mukherjee B, Sharma T, Halbe S, Jaisankar D, et al. Visual impairment in high flow and low flow carotid cavernous fistula. Sci Rep. 2019; 9(1):12872.

3. Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985; Feb. 62(2):248–56.

4. Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg. 1995; Feb. 82(2):166–79.

5. Chi CT, Nguyen D, Duc VT, Chau HH, Son VT. Direct traumatic carotid cavernous fistula: angiographic classification and treatment strategies. Study of 172 cases. Interv Neuroradiol. 2014; Jul-Aug. 20(4):461–75.

6. Cognard C, Gobin YP, Pierot L, Bailly AL, Houdart E, Casasco A, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology. 1995; Mar. 194(3):671–80.

7. Debrun GM. Angiographic workup of a carotid cavernous sinus fistula (CCF) or what information does the interventionalist need for treatment? Surg Neurol. 1995; Jul. 44(1):75–9.

8. Francis PM, Khayata MH, Zabramski JM, Spetzler RF. Carotid cavernous fistulae. Part 1: Presentation and features, in Carter LP, Spetzler RF (ed.) Neurovascular Surgery. New York: McGraw-Hill 1995. p. 1049-59.
9. Jung KH, Kwon BJ, Chu K, Noh Y, Lee ST, Cho YD, et al. Clinical and angiographic factors related to the prognosis of cavernous sinus dural arteriovenous fistula. Neuroradiology. 2011; 53(12):983–92.

10. Leone G, Renieri L, Enriquez-Marulanda A, Dmytriw AA, Nappini S, Laiso A, et al. Carotid cavernous fistulas and dural arteriovenous fistulas of the cavernous sinus: validation of a new classification according to venous drainage. World Neurosurg. 2019; Aug. 128:e621–31.

11. Malan J, Lefeuvre D, Mngomezulu V, Taylor A. Angioarchitecture and treatment modalities in posttraumatic carotid cavernous fistulae. Interv Neuroradiol. 2012; Jun. 18(2):178–86.

12. Ringer AJ, Salud L, Tomsick TA. Carotid cavernous fistulas: anatomy, classification, and treatment. Neurosurg Clin N Am. 2005; Apr. 16(2):279–95. viii.

13. Thomas AJ, Chua M, Fusco M, Ogilvy CS, Tubbs RS, Harrigan MR, et al. Proposal of venous drainage-based classification system for carotid cavernous fistulae with validity assessment in a multicenter cohort. Neurosurgery. 2015; Sep. 77(3):380–5. discussion 385.

14. Winn HR. Youmans and Winn Neurological Surgery. Philadelpia: Elsevier;2017. p. 3525–29.
Fig. 1.
(A) Angiogram in a post-traumatic CCF case showing communication of cavernous sinus with right ICA (high flow, direct) and right ECA. (B) Left ICA angiogram showing two sites of fistulous communication with cavernous sinus. CCF, carotid cavernous fistula; ICA, internal carotid artery; ECA, external carotid artery
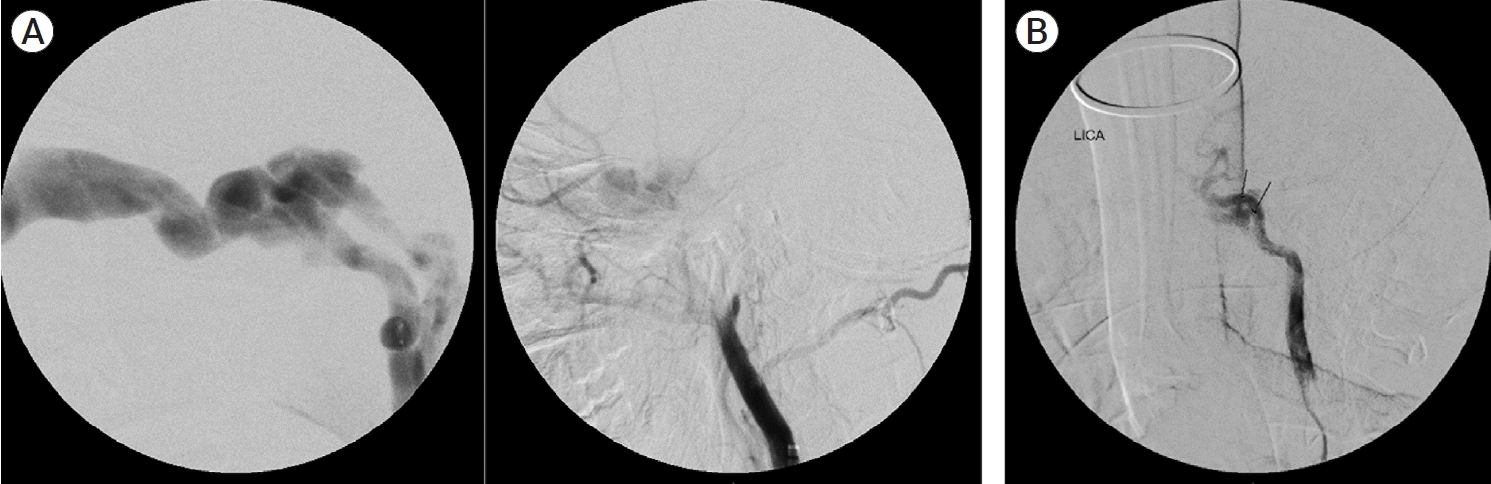
Fig. 2.
Diagrammatic representation of components of API-ACE classification scheme. (A) Sketch showing CCF in relation to ICA with predominantly anterior drainage (A1) into the superior ophthalmic vein (SOV). (B) CCF with predominantly posterior drainage (P1) into the superior and inferior petrosal sinus (SPS and IPS) in relation to the petrous bone. (C) CCF with inferior venous drainage (I1) into the pterygoid venous plexus through foramen in the skull base. (D) Arterial communication with the ICA A1- direct communication with ICA; A0- arterial communication via a branch of ICA. (E) CCF causing venous hypertension and reversal of blood flow in the cortical vein via superior sagittal and spheno-petrosal sinus (C1). (F) CCF arising from meningeal branch of external carotid artery (E1). CCF, carotid cavernous fistula; ICA, internal carotid artery
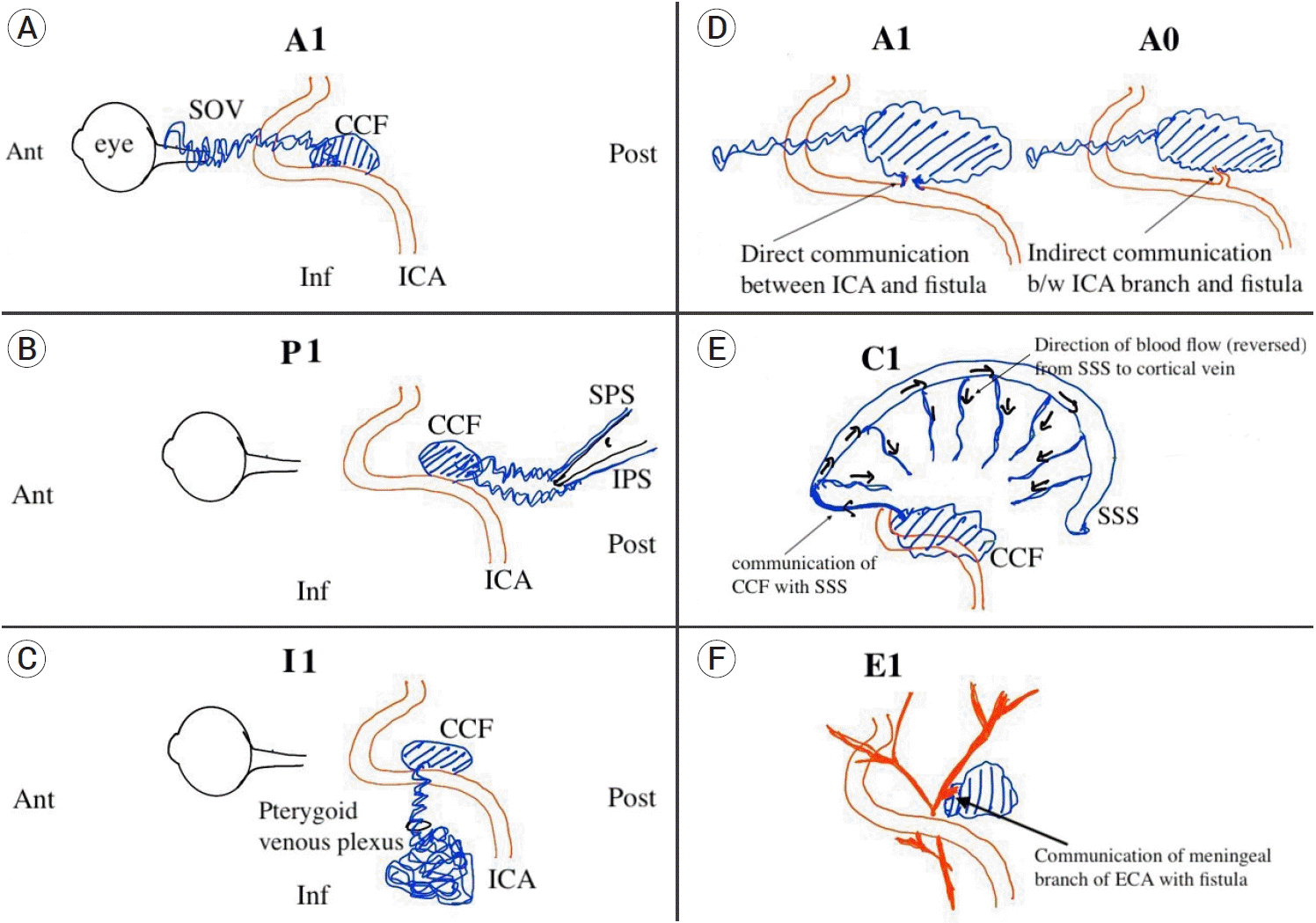
Fig. 3.
Schematic representation of API-ACE classification scheme and the distribution of cases in our series. ICA, internal carotid artery; ECA, external carotid artery
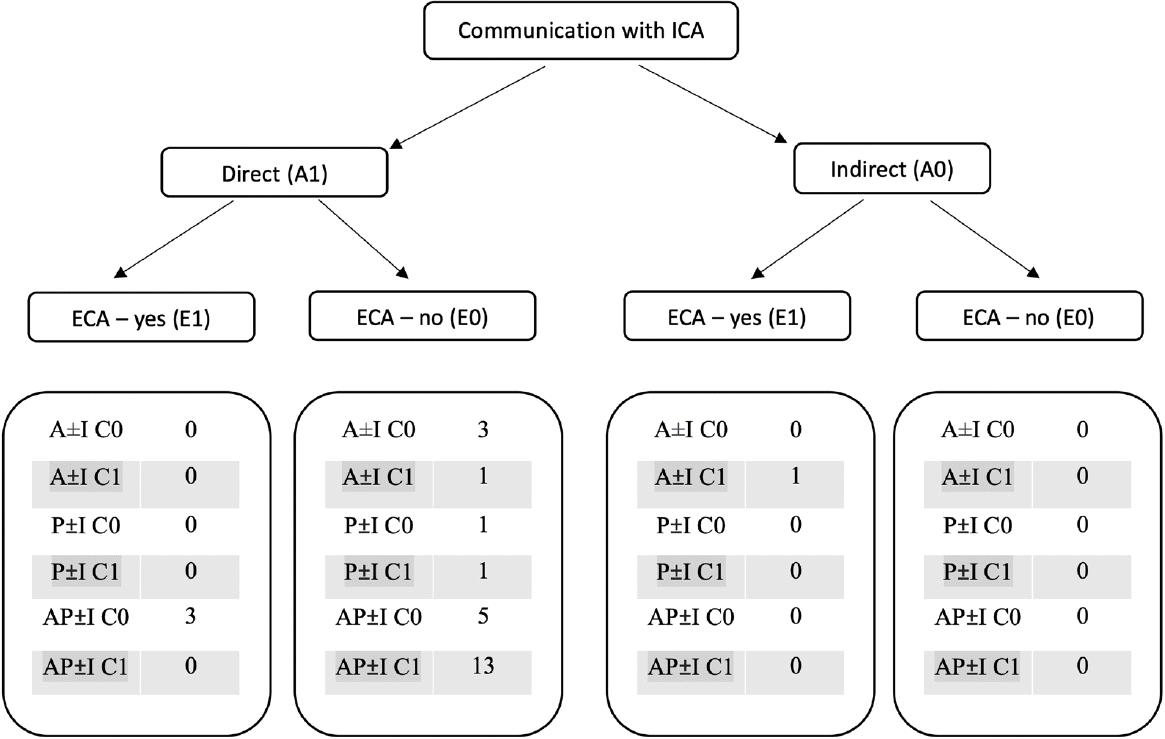
Fig. 4.
Direct, high flow CCF with no ECA contribution, with both anterior and posterior ± inferior drainage pattern. (A) Right ICA lateral angiogram showing API- A1 C0 E0 (B) Right ICA lateral angiogram showing API- A1 C1 E0. CCF, carotid cavernous fistula; ECA, external carotid artery; ICA, internal carotid artery
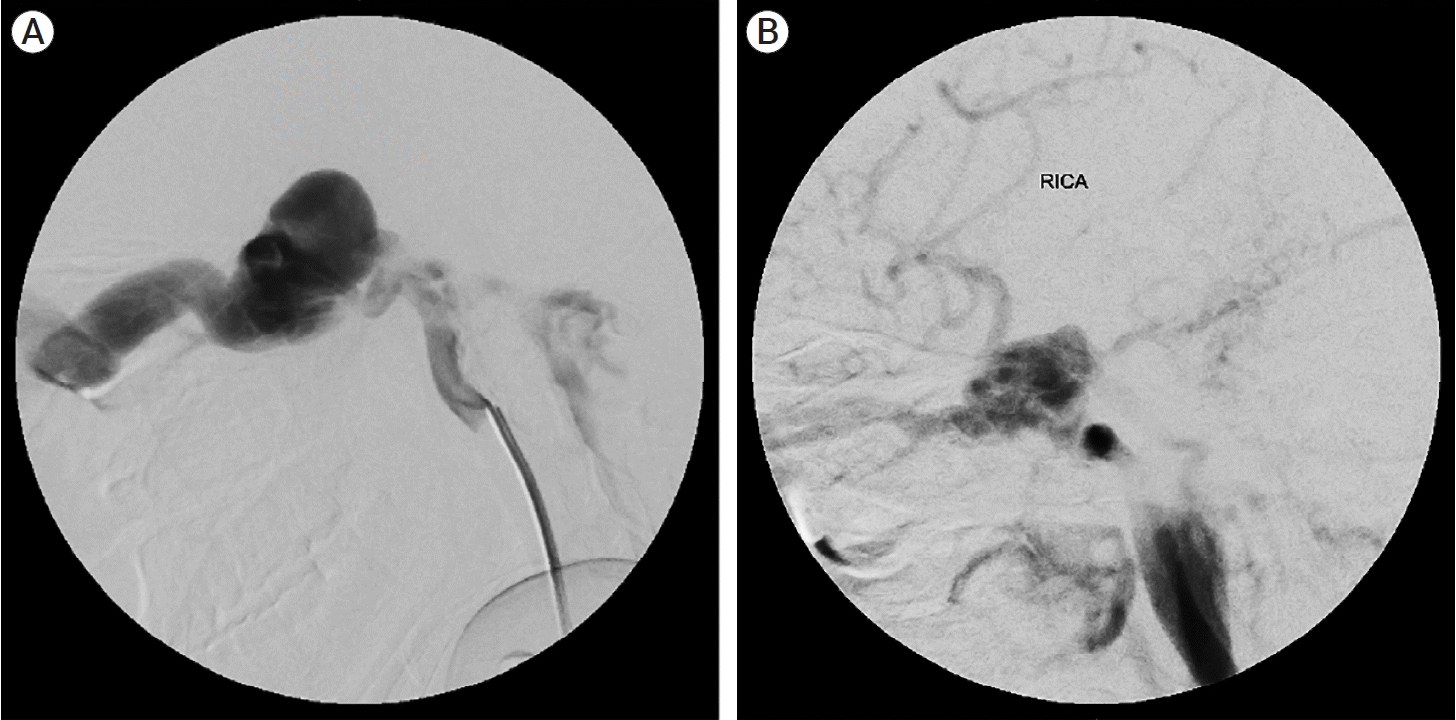
Fig. 5.
(A) Direct, high flow CCF with no ECA contribution, with predominant posterior venous drainage. Left ICA lateral angiogram showing PI- A1 C1 E0. (B) Direct, high flow CCF with no ECA contribution, with anterior venous drainage. Right ICA angiogram demonstrating A- A1 C0 E0 type of CCF. CCF, carotid cavernous fistula; ECA, external carotid artery; ICA, internal carotid artery
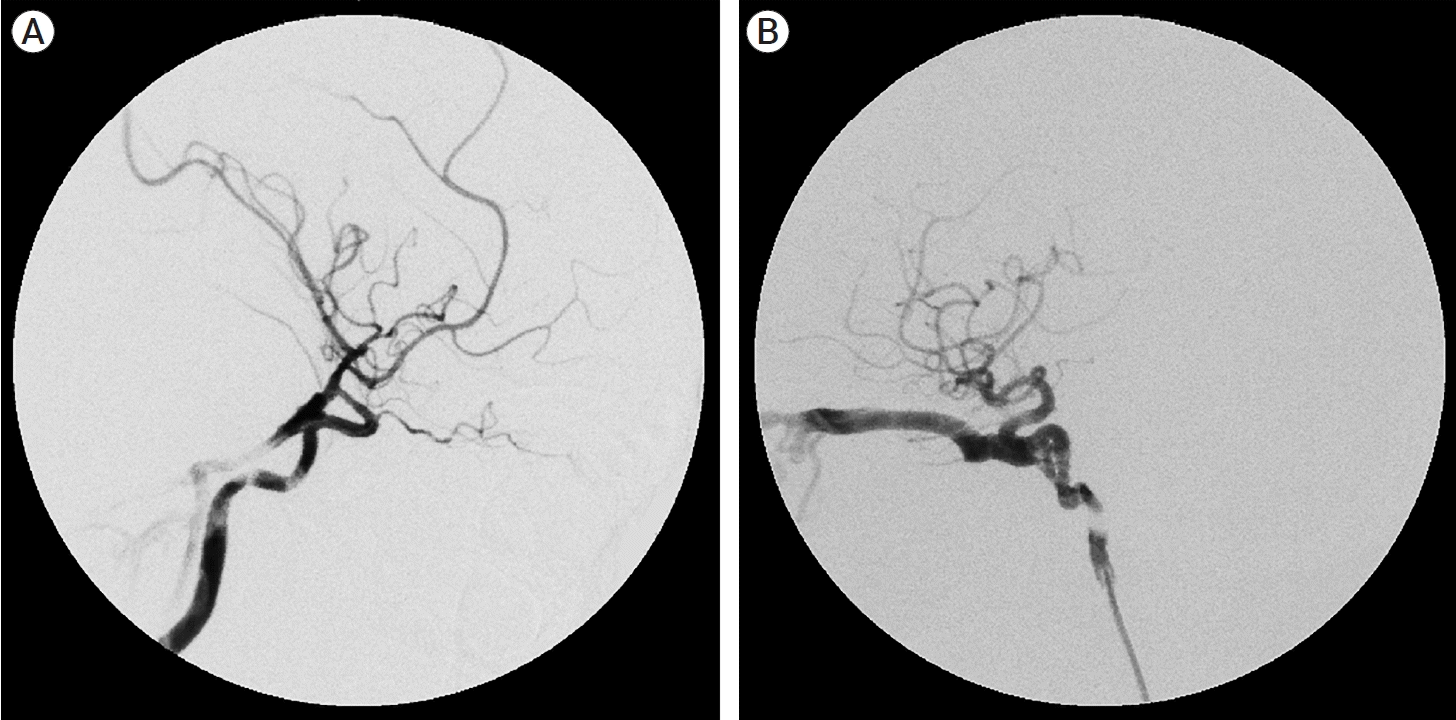
Table 1.
API-ACE classification for carotid-cavernous-fistula
Table 2.
Presenting symptoms
| Clinical features | Frequency |
|---|---|
| Proptosis | 28 |
| Chemosis | 28 |
| Orbital bruit | 25 |
| Ophthalmoplegia | 19 |
| Decreased visual acuity | 14 |
| Headache | 10 |
| Vertigo | 8 |
| Hearing loss | 2 |
| Unconsciousness | 6 |
Table 3.
Angiographic arterial anatomy
Table 4.
Venous architecture
Table 5.
CCF type according to Thomas classification
| CCF Type | Frequency |
|---|---|
| Type 1 | 0 |
| Type 2 | 2 |
| Type 3 | 0 |
| Type 4 | 2 |
| Type 5 | 24 |
Table 6.
Types of procedures performed
| Type of procedure | No. of procedure performed |
|---|---|
| Coiling | 19 |
| Balloon embolization | 6 |
| Coil + Balloon embolization | 4 |
| NBCA embolization | 2 |
| Coiling + NBCA embolization | 2 |
| Balloon + NBCA embolization | 1 |
Table 7.
Venous architecture of Barrow type A fistula (n=24)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download