This article has been
cited by other articles in ScienceCentral.
Abstract
Spinal dural arteriovenous fistula (SDAVF) is known for its ambiguous and various clinical presentations. Among these presentations, congestive myelopathy is one of the most common, yet it is challenging to correctly diagnose SDAVF at initial presentation. Several diseases present as myelopathy, including demyelinating diseases. Herein, we present two cases of congestive myelopathy due to SDAVF presenting to the emergency room (ER) with progressive quadriparesis. Even though the patients had a proper magnetic resonance imaging (MRI) examination from the initial presentation, there was a delay in making a final diagnosis. Both patients’ clinical presentation and MRI mimicked central nervous system (CNS) demyelinating disease initially, and a more thorough examination revealed SDAVF. Such a delay in diagnosis can result in more neurological deterioration and may result in more sequelae. Hence, SDAVF should always be considered as a differential diagnosis when examining patients with myelopathy.
Go to :

Keywords: Dural arteriovenous fistula, Congestive myelopathy, Demyelinating diseases, Neuromyelitis optica, Multiple sclerosis, Quadriplegia
INTRODUCTION
Spinal dural arteriovenous fistula (SDAVF) is the abnormal drainage of a dural arteriovenous malformation (AVM) into the perimedullary venous plexus [
1,
3,
4,
7]. The veins become engorged and are unable to adequately drain the influx of blood, resulting in venous hypertension and infarction. SDAVF can induce congestion of blood flow and result in medullary and spinal cord edema [
3]. The most common symptom is myelopathy, but delayed diagnosis due to symptom variability in the early stage and difficulty in early detection could result in neurologic sequelae [
3,
7]. In atypical clinical situations, vascular myelopathy is easily neglected and other neurological diagnoses can be made, such as demyelinating disease, including acute transverse myelitis (TM), multiple sclerosis (MS), or neuromyelitis optica (NMO) [
2]. Herein, we present two cases of SDAVF that could have been easily misdiagnosed as other demyelinating diseases.
Go to :

DESCRIPTION OF CASES
Case 1
A 67-year-old man visited the emergency room (ER) at night with progressive quadriparesis, sensory abnormality, and voiding difficulty that started a day before his visit. He had been treated for hypertension and diabetes. Moreover, the patient had a history of suspected transient ischemic attack when he had transient paraparesis and paraparesthesia in his lower extremities several years ago. He also experienced transient hiccups and transient paresthesia in his upper extremity one year before the ER visit. Upon neurological examination in the ER, cranial nerve functions were intact: there was right-dominant quadriparesis (Medical Research Council (MRC) grade 2-3 on the right side and MRC grade 4 on the left side) and hyperactive deep tendon reflex (DTR). Hypesthesia was observed on the right side, below the T10 dermatome. Initial brain magnetic resonance imaging (MRI) revealed a diffuse signal change from the medulla to the upper cervical spinal cord (
Fig. 1A). Complete blood count (CBC) was normal as revealed by laboratory testing and cerebrospinal fluid (CSF) examination showed elevated protein (83 mg/dL) without pleocytosis (WBC, 0) and normal glucose level (CSF, 55 mg/dL; serum, 91 mg/dL). Initial spine MRI showed an intramedullary high signal from the cervical spinal cord of the cervicomedullary junction to the T1 spinal cord (
Fig. 1B). However, we failed to recognize small abnormal vessel flow voids on the dorsal pial surface from the cerebellum to the T-spine on the initial MRI (
Fig. 1B). The patient had recurring neurologic deficits, such as transient paraparesis, paraparesthesia, and hiccups. We initially suspected central nervous system (CNS) demyelinating disease such as MS or NMO because the patient’s MRI showed high signal intensity in the spinal cord and he had recurring neurologic symptoms. Hence, the patient was initially treated with steroid pulse therapy and showed mild improvement overnight, but quadriparesis persisted.
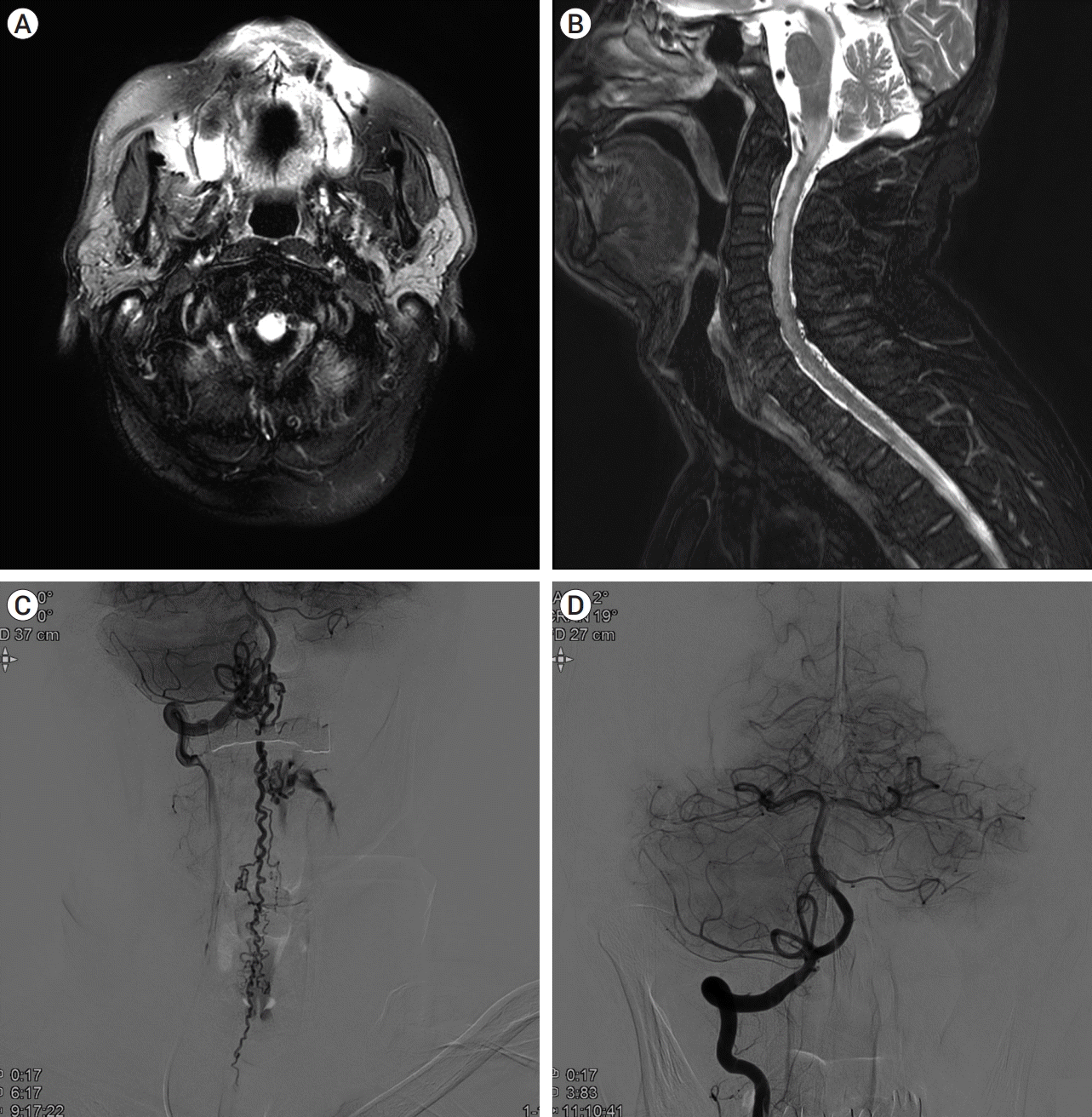 | Fig. 1.
MRI and DSA of Case 1.
Initial brain MRI showing increased signal intensity at the medulla in fluid-attenuated inversion recovery (FLAIR) imaging (A). C-spine MRI shows multiple small abnormal vessel flow voids on the dorsal pial surface from the cerebellum to the T-spine in T2 weighted image, and intramedullary high signal and a peripheral low signal from the cervicomedullary junction to T1 with cord swelling (B). DSA image confirms AV shunt at the C1 level with prominent perimedullary draining vein along the anterior and posterior surface of the spinal cord (C). Shunt feeder from the right. The V3-V4 junction was embolized, and the final angiogram shows the disappearance of the AV shunt (D). MRI, magnetic resonance imaging; DSA, Digital subtraction angiography; AV, arteriovenous

|
After radiologic consultation the following morning, the patient was diagnosed with SDAVF. Nevertheless, the location of the feeding artery to the SDAVF was not clear, and we performed C-spine MR angiography and conventional angiography with microcatheter: an arteriovenous (AV) shunt between the vertebral artery and perimedullary venous drainage along the anterior and posterior surface of spinal cord was revealed (
Fig. 1C), and we successfully embolized two feeding arteries from the right ventricle V3-V4 junction using 25% n-Butyl Cyanoacrylate (NBCA) (
Fig. 1D). The patient’s symptoms improved after the procedure. The patient’s bilateral upper extremities and left lower extremity showed an MRC grade of 5 on both sides and the right leg showed an MRC grade of 4 upon discharge.
Case 2
A 53-year-old man with underlying gout and dyslipidemia visited the emergency room with progressive paraparesis, dysarthria, and dysphagia, and symptoms beginning with general weakness and myalgia four days before his visit. General weakness and myalgia did not respond to conservative treatment and gradually progressed to paraparesis. Upon neurologic examination in the ER, the motor power of his lower extremities was MRC grade 1 with normoactive knee jerk. He complained of paresthesia below the T6 dermatome. Initial brain MRI showed a high signal change in the lower medulla (
Fig. 2A). Laboratory tests revealed leukocytosis (WBC, 15300/mm
3). A cerebrospinal fluid study was performed in the ER and showed no signs of infection (WBC, 0). Protein levels were within the normal range (55 mg/dL), and glucose levels were normal (CSF, 78 mg/dL; serum, 135 mg/dL). Since we had recently observed SDAVF in Case 1, we carefully looked for abnormal vessel flow void on MRI, but it was not easy to recognize. Suspecting a CNS demyelinating disease, such as NMO or MS, we started steroid pulse therapy (prednisolone 1 g IV therapy) for the first five days, but there was no improvement or worsening of the symptoms. Eventually, an additional C-spine MRI revealed an enhancing serpentine vessel in the ventral and dorsal surfaces of the spinal cord at the C3-7 level (
Fig. 2B). Conventional angiography with micro-catheter revealed a dural arteriovenous fistula from the right posterior meningeal artery (
Fig. 2C). After we effectively embolized the feeding artery with 20% NBCA (
Fig. 2D), his symptoms improved gradually, and the motor grade of both lower legs improved to MRC grade 4 when he was discharged from the rehabilitation department.
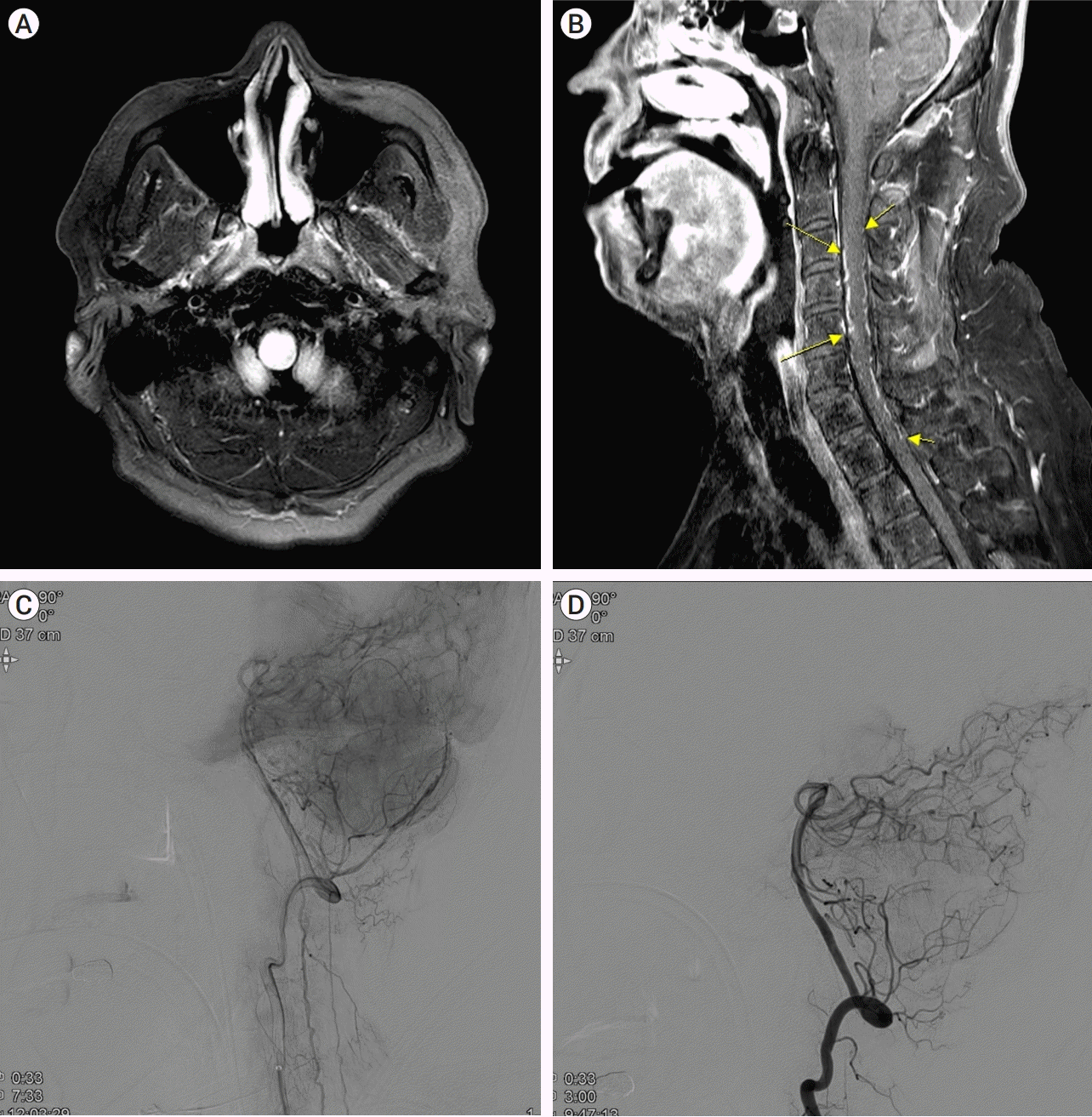 | Fig. 2.
MRI and DSA of Case 2.
Initial brain MRI shows T2 high signal intensity in the medulla and cervical cord without diffusion restriction (A). Along with the high signal intensity at the medulla, cervical T1 enhanced image demonstrates abnormal enhancing serpentine vessels in the ventral and dorsal surfaces of the spinal cord from the C3 to C7 level (B, yellow arrows). DSA reveals dural AVF at posterior Rt, meningeal angiography (C). Embolization of the Rt. The posterior meningeal artery was performed, and the final angiogram shows occlusion of the dural AVF (D). MRI, magnetic resonance imaging; DSA, Digital subtraction angiography; AVF, arteriovenous fistula

|
Go to :

DISCUSSION
SDAVF is a rare and heterogeneous group of spinal vascular malformations [
5], with an incidence of 5-10 cases per million a year. It occurs mainly in men, the older population (>40 years), or those with history of back surgery or trauma [
7]. Clinical presentation could be vague. Due to phenotypic variability, it takes a long time for patients to obtain precise diagnoses, and treatment is often delayed, resulting in more neurologic sequelae. Andrew et al. reported that 16 out of 46 patients had been misdiagnosed initially, and it took a significantly long time to diagnose correctly from symptom onset [
7]. In the misdiagnosed group, initial diagnoses include spinal stenosis, tumor, Guillain-Barre syndrome, MS, and NMO [
7]. In this study, demyelinating disease was initially suspected, although the possibility was low due to the absence of CSF pleocytosis. Various diseases should be considered in patients with neurological abnormalities of myelopathy, including demyelinating disease, spinal infarction, and arteriovenous malformation. Since there is a limitation in obtaining detailed images in the ER, it is easy to misdiagnose as other diseases if the radiologic findings are not obvious.
Angiography is the most accurate method for the radiologic diagnosis of SDAVF, but MRI is more accessible. SDAVF shows three characteristics on MRI: spinal cord hyperintensity signal on T2-weighted image (WI), enlarged pial flow void around the spinal cord on T2WI, and contrast enhancement on T1WI [
5]. Among these, enlarged pial flow void is better measured in T2WI, and it is not only easier to examine in the ER setting but also more accessible in patients who cannot use contrast enhancement. The enlarged blood vessels show serpentine-like filling defects. In Case 1, such flow voids were observed, but in Case 2, they were not visible in the initial imaging examination even though we paid more attention after observing them in the patient from Case 1. Digital subtraction angiography (DSA) is the gold standard for diagnosing SDAVF. Especially in cervical arteriovenous fistula (AVF), four-vessel angiography should be considered to find the AVF feeder. In our cases, we confirmed that the vertebral artery was a feeder of the SDVF through four-vessel angiography.
Embolization of SDAVFs is the most common and effective treatment. In this study, both cases were treated with embolization, and their clinical symptoms improved. However, it takes time to go through DSA and embolization, and the patient’s neurological symptoms may progress during the delay. As mentioned earlier, medical treatment such as high-dose steroids precedes an accurate diagnosis because of the time delay. Silvia et al. reported that a patient with SDAVF was misdiagnosed with myelitis and rapidly worsened with the use of intravenous steroids [
6]. Using steroids with saline might have interfered with venous circulation around the spinal cord [
6]. However, there is still a lack of large-scale studies on the harm of steroid use in SDAVF. In our cases, both patients were initially treated with high-dose steroids and showed slight clinical improvement. There is not enough evidence since the study observed only two cases; however, alternative treatment options during the diagnosis process should be further studied.
Go to :

CONCLUSIONS
SDAVF has an ambiguous clinical feature with characteristic MRI findings; its diagnosis should be accompanied by careful examination and clinical experience. As the diagnosis is delayed, the neurological deterioration in the patient may progress. Therefore, we hope that this study will serve as an opportunity to consider SDAVF in the differential diagnosis of patients with myelopathy.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download