INTRODUCTION
CASE PRESENTATION
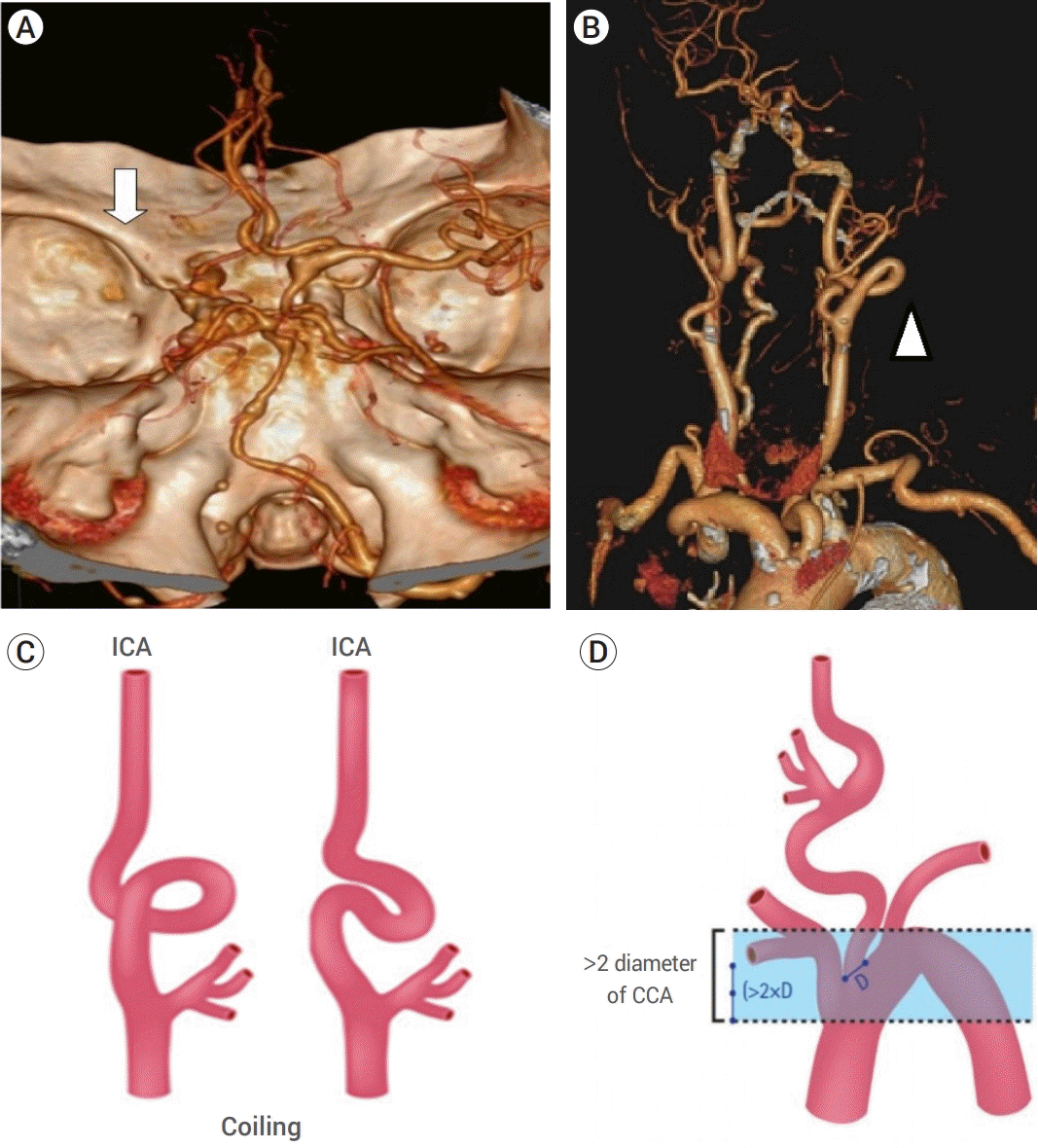 | Fig. 1.The anatomic features are known to be unfavorable for endovascular procedures. (A) Shows computed tomographic angiography of the brain (white arrow) shows flow arrest caused by left middle cerebral artery occlusion. (B) Shows the aortic arch and the curvature of the internal carotid artery (ICA) (head of the white arrow) in the patient studied. (C) Shows the “coiling” feature of proximal ICA with extreme tortuosity, and (D) shows the vertical distance between the innominate artery and the top of the aortic arch, which was greater than twice the diameter of the left common carotid artery [2]. |
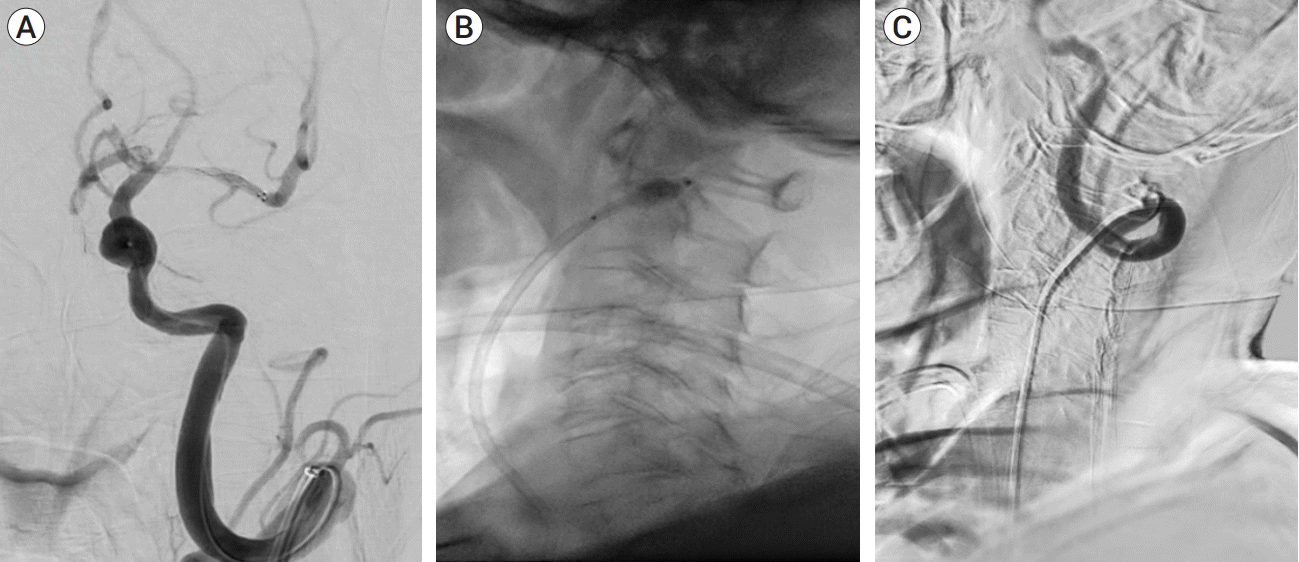 | Fig. 2.Cerebral angiography in the patient studied. (A) Shows the anteroposterior view of cerebral angiography after stent deployment for stent retrieval. Because of the recanalization of the left middle cerebral artery, distal flow is visible. However, re-occlusion occurred during stent retrieval. (B) Shows the lateral view of the balloon guide catheter (BGC) in the proximal part of the coiling segment in the left ICA. The sudden deflation of the BGC failed, and there was prolonged arrest of intracerebral flow. (C) Successful deflation was achieved, and intracerebral flow restored following a series of processes involving the excision of a proximal portion of the BGC and the passage of cutting balloon through the BGC. |
Procedure for mechanical thrombolysis
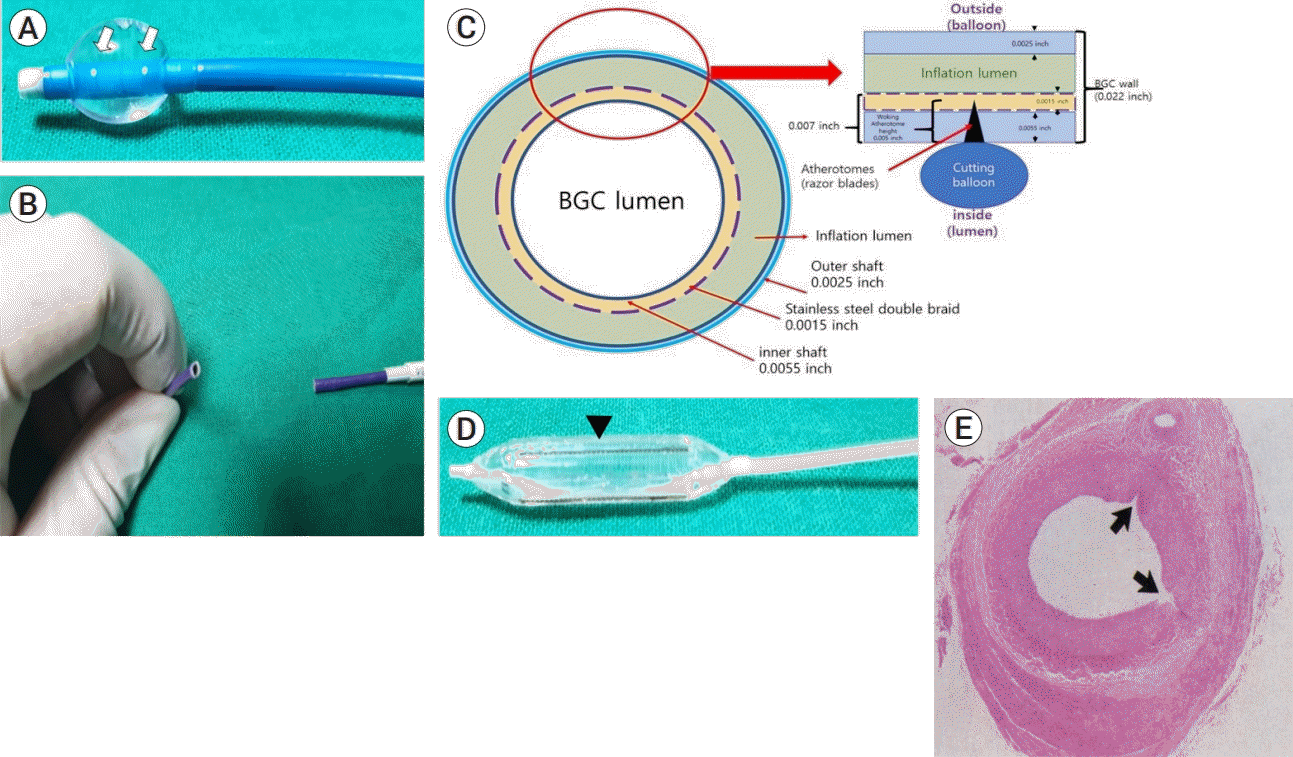 | Fig. 3.The structural feature of the balloon guide catheter (BGC) and cutting balloon. (A) Is an inflated BGC; it shows leakage of the contrast media from the two white dots (white arrows) above the region in which the balloon is inflated. (B) Shows the excised surface of the BGC, which is relatively clean, and the structure has not been crushed or deformed. (C) Shows the cross-sectional structure of the excised BGC. The balloon of the BGC is located outside the 0.022-inch catheter wall, which consists of an outer shaft, stainless metal braid and inner shaft with thickness of 0.0025-, 0.0015- and 0.0055-inches, respectively. Because, the thickness of the catheter from the lumen to the inflation lumen is 0.007 inches, the 0.005-inch blade of the cutting balloon does not reach the balloon of the BGC, or the inflation lumen. (D) Shows three to four razor blades (at the heads of the black arrows) present on the surface of the cutting balloon. (E) Shows a cross-section of the transverse microincision made by the cutting balloon on the vessel lumen (black arrow) [4]. The BGC wall was expected to be damaged by the blades of the cutting balloon, resulting in the deflation of the balloon. |
Troubleshooting
DISCUSSION
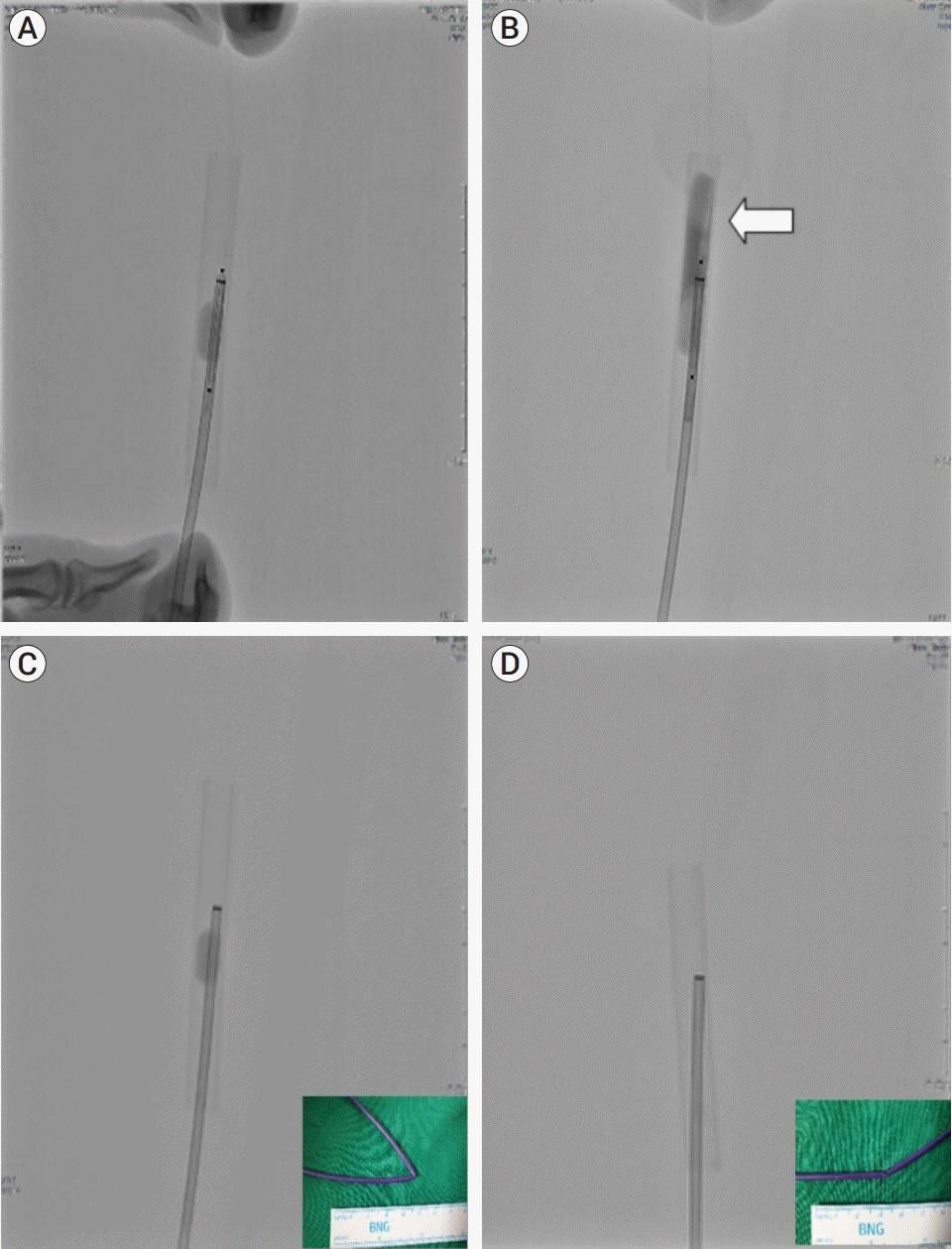 | Fig. 4.The methodological analysis of our experience with balloon guide catheter (BGC) balloon deflation failure, which was confirmed by a reproducible experiment under similar conditions. (A) Shows the introduction of the cutting balloon in the inflated BGC. (B) No deflation was observed in the BGC balloon, although sufficient pressure was applied for the cutting balloon to rupture the BGC. The contrast media is leaking due to a rupture in the cutting balloon (white arrow). (C) Shows the BGC inflated inside a rubber tube. The balloon did not deflate despite the excision of the proximal portion of the BGC. This may be attributed to the luminal collapse caused by the flexure in the catheter. (D) After excising the proximal portion of the BGC and straightening the kink in the flexure of the catheter, luminal patency is obtained, and deflation occurs automatically within 30 s. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download