Abstract
Background
Thyroxine-binding globulin (TBG) is a major transporter protein for thyroid hormones. The serpin family A member 7 (SERPINA7) gene codes for TBG, and mutations of the SERPINA7 gene result in TBG deficiency. Although more than 40 mutations have been reported in several countries, only a few studies of TBG deficiency and SERPINA7 gene mutation have been performed in Korea. The aim of this study is to review the clinical presentations and laboratory findings of patients with TBG deficiency and to investigate the types of SERPINA7 gene mutation.
Methods
Five unrelated Korean adults with TBG deficiency attending endocrinology clinic underwent SERPINA7 gene sequencing. Four patients harbored a SERPINA7 gene mutation. Serum thyroid hormones, anti-microsomal antibodies, and TBG were measured. Genomic DNA was extracted from whole blood. All exons and intron-exon boundaries of the TBG gene were amplified and sequencing was performed.
Results
Two patients were heterozygous females, and the other two were hemizygous males. One heterozygous female had coexisting hypothyroidism. The other heterozygous female was erroneously prescribed levothyroxine at a local clinic. One hemizygous male harbored a novel mutation, p.Phe269Cysfs*18, which caused TBG partial deficiency. Three patients had the p.Leu372Phefs*23 mutation, which is known as TBG-complete deficiency Japan (TBG-CDJ) and was also presented in previous mutation analyses in Korea.
Conclusion
This study presents four patients diagnosed with TBG deficiency and provides the results of SERPINA7 gene sequencing. One novel mutation, p.Phe269Cysfs*18, causing TBD-partial deficiency and three cases of TBG-CDJ were demonstrated. It is necessary to identify TBG deficiency to prevent improper treatment. Also, sequencing of the SERPINA7 gene would provide valuable information about the TBG variants in Korea.
Thyroxine-binding globulin (TBG) is a major thyroid hormone binding protein that binds to approximately 70% of triiodothyronine (T3) and 75% of thyroxine (T4) in serum [1-4]. TBG, a 54-kDa glycoprotein with a single chain of 395 amino acids, is synthesized in the liver. It is encoded by four exons in single gene called serpin family A member 7 (SERPINA7) which is located on the long arm of the X-chromosome (Xq22.2) [5]. TBG mutations have three phenotypes of TBG-complete deficiency (TBG-CD), TBG partial deficiency (TBG-PD), and TBG excess (TBG-E). TBG-CD is a rare condition harboring undetectable TBG concentration with a frequency of 1 in 15,000 newborns. TBG-PD is the most common phenotype among the TBG deficiencies with a frequency of 1 in 4,000 newborns [6-8]. The affected patients present clinically euthyroid status but show abnormal results in thyroid function tests (TFT) which can lead to misdiagnoses and improper treatment. Because TBG mutations are inherited in X-linked fashion, TBG deficiency is fully expressed in hemizygous males and homozygous females and less expressed in heterozygous females whose TBG concentration can overlap with the normal range [9].
To date, 49 mutations in the SERPINA7 gene have been reported to cause TBG deficiency [10-14]. In Korea, reports of TBG deficiency mainly discuss neonates and pediatric patients, and only a few mutation analyses of the SERPINA7 gene have been conducted. Park et al. [15] performed a mutation analysis in two neonates and revealed a single nucleotide deletion of codon 352 in exon 4, which is a prevalent variant in the Japanese population, called TBG-complete deficiency Japan (TBG-CDJ) [15,16]. A mutation analysis of an 11-year-old boy with TBGCD by Baek et al. [17] also found the TBG-CDJ mutation. Pappa et al. [14] provided a summary of all TBG mutations reported to date including a Korean variant named TBG-PDKa that caused TBG-PD. However, because TBG-PDKa was not published in the literature, clinical information such as patient age, sex, habitation, comorbidities, and medication are unknown. In this study, we review the clinical presentations and laboratory findings of adult patients with TBG deficiency in Korea, and investigate the types of SERPINA7 gene mutation through direct sequencing.
Five unrelated Korean patients with TBG deficiency attending endocrinology clinic at Samsung Medical Center underwent SERPINA7 gene sequencing with informed consents between June 2021 and March 2022. Four of the five patients, two males and two females, harbored SERPINA7 gene mutations, and one of them presented a novel mutation. This study was approved by the Institutional Review Board of Samsung Medical Center (SMC-IRB 2021-06-134).
Total T4, total T3, free T4, free T3, thyroid stimulating hormone (TSH), anti-microsomal antibody (AMA) and TBG were measured using the immunoradiometric assay (IRMA), radioimmunoassay (RIA), and electrochemiluminescence immunoassay (ECLIA) techniques.
Blood specimens were collected from the study patients to for SERPINA7 sequencing. Using a Wizard genomic DNA purification kit, genomic was extracted from whole blood in accordance with the manufacturer’s instructions (Promega, Madison, WI, USA). All intron-exon boundaries and exons of the SERPINA7 gene were amplified, and direct sequencing was conducted using an ABI Prism 3100 GeneticAnalyzer (Applied Biosystems, Waltham, MA, USA) with a BigDye terminator cycle sequencing-ready reaction kit (Applied Biosystems). The primer sets used for direct sequencing are shown in Supplemental Table S1. The nucleotides and corresponding protein sequences were represented in accordance with the reference sequences of NM_000354.6 and NP_000345.2, respectively. The pathogenicity of the variants was analyzed with the Single Nucleotide Polymorphism Database (dbSNP) 147, Clinvar, and Human Gene Mutation Database (HGMD). The population frequency was acquired through gnomAD v.2.1.1 and the Korean Reference Genome Database (KRGDB, accessed on October 15, 2021). The variants were classified into five groups following the American College of Medical Genetics and Genomics/Association for Molecular Pathology (ACMG/AMP) guidelines.
This female patient was 47-year-old when she first visited endocrinology clinic of Samsung Medical Center after long-term fluctuation in thyroid function. The patient had been diagnosed with hypothyroidism at a local clinic in 2018. Otherwise she had no remarkable medical history while her mother and sister had hypothyroidism. In the 2nd year of taking levothyroxine, her T3 level remained low, and her levothyroxine dosage was increased. Fatigue, palpitation, hand tremor and excessive weight loss of 10 kg followed the dosage increase. Failure to adjust appropriate dosage of levothyroxine led the patients to our endocrinology clinic on November 3, 2020. The serum free T4 and TSH levels reported at the local clinic shortly before her visit to our clinic were 2.09 ng/dL (normal range, 0.79 to 1.86) and 0.03 μIU/mL (normal range, 0.3 to 6.00), respectively, while on 88 μg of levothyroxine. No abnormal feature was observed in physical examination, thyroid ultrasonography, or thyroid scintigraphy. The AMA, thyroglobulin antibody, and antiTSH receptor antibody were all within normal range. Measurement of TBG showed a decreased level of 5.35 μg/mL (normal range, 10.90 to 43.20). Levothyroxine was gradually tapered off. About 3 months after discontinuation of levothyroxine, she had no signs or symptoms of hypothyroidism, and her free T4, TSH, and total T3 were 1.16 ng/dL (normal range, 0.79 to 1.86), 6.58 μIU/mL (normal range, 0.3 to 6.00), and 42.2 ng/dL (normal range, 76 to 190), respectively. Upon diagnosis of TBG deficiency rather than hypothyroidism, sequencing of the SERPINA7 gene was conducted. As a result, a 1-bp deletion (c.1114del) in exon 5 was detected.
A 44-year-old male presented with decreased levels of total T3 of 64 ng/dL (normal range, 80 to 200) and total T4 of 3.7 μg/dL (normal range, 5.1 to 14.1) during a routine health examination in 2001 at Samsung Medical Center. His TSH was 1.14 μIU/mL (normal range, 0.3 to 6.00). The patients had no medical or family history. He did not present symptoms of hypothyroidism or thyrotoxicosis, and no abnormal findings were observed in physical examination. Thyroid ultrasonography revealed nothing significant. His AMA, thyroglobulin antibody, and anti-TSH receptor antibody levels were all within the normal range. Thereafter, decreased levels of total T3 and T4 were consistently observed in TFTs conducted every 1 to 2 years. Elevated free T4 without any symptoms or signs of thyrotoxicosis was observed episodically, and that led him to visit our endocrinology clinic in 2017, when he was 61-year-old. His TBG level was 1.42 μg/mL (normal range, 10.90 to 43.20), and under the diagnosis of TBG deficiency, TFTs have been performed every year without any treatment. The result of sequencing of SERPINA7 gene was a 1-bp deletion (c.1114del) in exon 5.
A 41-year-old female visited a local clinic in 2011 because of anterior neck discomfort and eyelid swelling. Testing of her thyroid function revealed hypothyroidism with TSH above 100 μIU/mL (normal range, 0.3 to 6.00) and decreased free T4 of 0.18 ng/dL (normal range, 0.79 to 1.86). She visited our endocrinology clinic after initiation of levothyroxine 100 μg. We adjusted her dosage to 75 μg. TSH was suppressed to 0.059 μIU/mL (normal range, 0.3 to 6.00), whereas total T3 level stayed low, with a value of 74.01 ng/dL (normal range, 76 to 190). Her AMA and thyroglobulin antibody were both positive with values of 133.1 U/mL (normal range, 0 to 60) and 835.3 U/mL (normal range, 0 to 60), respectively. Thyroid ultrasonography showed diffuse parenchymal changes suggestive of thyroiditis. She did not have any other medical history except hypothyroidism. Her son was also diagnosed with hypothyroidism. While being treated with 50 μg of levothyroxine, her total T3 level was constantly low, but her free T4 and TSH levels were in the normal ranges. Testing for TBG showed a decreased concentration of 5.47 μg/mL (normal range, 10.90 to 43.20), which produced a diagnosis of hypothyroidism combined with TBG deficiency. The SERPINA7 gene sequencing revealed a 1-bp deletion (c.1114del) in exon 5.
A 61-year-old male showed low total T3 in the TFTs of his routine health check-ups beginning in 2013, though his free T4 and TSH levels were in the normal ranges. Consequently, he was referred to our endocrinology clinic in 2021. He did not have any medical or family history, and he was not on any medication. Physical examination and review of system revealed no abnormal findings. Thyroid ultrasonography also presented normal thyroid volume and parenchyma. At his first visit to our endocrinology clinic, his TSH, total T3, and free T4 were 2.62 μIU/mL (normal range, 0.3 to 6.00), 48.8 ng/dL (normal range, 76 to 190), and 1.16 ng/dL (normal range, 0.79 to 1.86), respectively. His AMA, thyroglobulin antibody, and anti-TSH receptor antibody levels were all within normal ranges. His TBG level was low as 5.16 μg/mL (normal range, 10.90 to 43.20), which led to a diagnosis of TBG deficiency. Sequencing of SERPINA7 gene showed a 2-bp deletion (c.806_807del) in exon 3.
The results of sequencing of the SERPINA7 gene of the four study patients are shown in Fig. 1. In heterozygous female patient 1 and 3, and in hemizygous male patient 2, a 1-bp deletion (c.1114del) in exon 5 was detected. The deletion was predicted to cause a frameshift and generate a premature stop codon 23 amino acids downstream of the variant position [p.(Leu372Phefs*23)]. Codon 372 occurs in the middle of the last coding exon (exon 5), meaning that it would escape nonsense-mediated mRNA decay. Because the variant is predicted to remove less than 10% of the protein product (22/415 amino acids), pathogenic and very strong (PVS1) was applied at a moderate evidence level. The allele frequency in the control database (0.0002 in gnomAD East Asian) was below the maximum credible population allele frequency (0.002) calculated using the alleleFrequencyApp tool [18] with 1:4,000 for prevalence and setting allelic heterogeneity to 0.1, genetic heterogeneity to 1, and penetrance to 50% [14,19]. This variant has previously been reported in other patients affected with complete TBG deficiency in the Japanese population [16,20]. Therefore, this frameshift variant was classified as a likely pathogenic variant (PVS1_moderate+pathogenic and moderate [PM2]+pathogenic and strong [PS4]) [21].
In patient 4, sequencing of SERPINA7 gene revealed a hemizygous 2-bp deletion (c.806_807del) in exon 3 (Fig. 1). The deletion was predicted to cause a frameshift and generate a premature stop codon 18 amino acids downstream of the variant position, resulting in nonsense-mediated decay [p.(Phe269Cysfs*18)]. This variant was not found in control databases, such as the gnomAD (East Asian population) and KRGDB, nor has it been previously reported. Therefore, this novel frameshift variant was classified as a likely pathogenic variant (PVS1+PM2) [21].
The results of the baseline characteristics, TFTs, TBG, thyroid autoantibodies, and direct sequencing of the SERPINA7 gene of the four study patients are provided in Table 1. At their first visit to our endocrinology clinic, the patients ranged from 47 to 64 years of age. In terms of mutations, patient 1 and 3 were heterozygous females, and patient 2 and 4 were hemizygous males. Patient 1, 2, and 3 harbored a known mutation, p.Leu372Phefs* 23 (TBG-CDJ), which is a prevalent mutation type in Japan. Patient 4 presented a novel mutation, p.Phe269Cysfs*18, causing TBG-PD. Only patient 3 had coexisting hypothyroidism. Patient 1 and 3 had a family history of hypothyroidism, but additional sequencing of the family members was not performed.
In this study, direct sequencing of the SERPINA7 gene was conducted in five unrelated adults with TBG deficiency from a tertiary center in Korea. Four of those patients harbored SERPINA7 gene mutation, one novel mutation of p.Phe269Cysfs*18 and three known mutations of p.Leu372Phefs*23. The affected females, patient 1 and 3, were heterozygous for the mutation. Patient 4, a hemizygous male had a novel mutation and presented a TBG level of 5.16 μg/mL, which is similar to the levels of heterozygous females, suggesting that the novel mutation p.Leu372Phefs*23 caused TBG-PD.
This study is, to our knowledge, the first published SERPINA7 gene mutation analysis reporting a novel mutation other than TBG-CDJ in Korea and the first to be performed exclusively on unrelated adults in Korea. As summarized in Table 4, nine studies reporting TBG deficiency were previously conducted, and four of them performed SERPINA7 gene sequencing, of which the results were all TBG-CDJ [15,17,22-28]. A summarized report by Pappa et al. [14] about all genetically identified TBG mutations contained a variant of TBG-PD caused by a G to A transition of codon 74 in exon 1 of the SERPINA7 gene which replaced glutamic acid to lysine. That mutation was designated as a Korean variant (PDKa) in that study; however, it was impossible for us to verify any reference among the published papers.
Considering that TBG-CD is defined as undetectable TBG (below 5 μg/mL or 0.003% the average normal) [29] in a hemizygous patient where only mutant allele is expressed, the new p.Phe269Cysfs*18 mutation is regarded to cause TBG-PD. However, it is unusual that this mutation resulted in TBG-PD rather than TBG-CD. Most cases of TBG-CDs are caused by nucleotide deletion like the novel mutation in this study, and point mutation is the only reported mechanism to cause TBG-PD so far [14]. Notably, the locus of this novel mutation is between those of the two mutations causing TBG-CD, TBG-CD Poland and TBG-CDJ [16,30]. Meanwhile, some cases of TBG-CDs are also caused by point mutations causing protein truncation [31-33]. Other factors such as protein folding or secretion can also affect the phenotype, so a protein structural analysis will help to verify the mechanism of the discordance in the novel mutation.
The natural course until the diagnosis of TBG deficiency in our study subjects was well described here. Patient 1 was erroneously diagnosed with hypothyroidism and was on levothyroxine, and she eventually was tapered off levothyroxine after being diagnosed with TBG deficiency. Patient 3 was initially diagnosed with hypothyroidism, but because TSH was suppressed as the levothyroxine dosage was increased but T3 remained consistently low, TBG deficiency was suspected. Generally, TBG deficiency is suspect when total thyroid hormones are low while free T4 and TSH are normal [14]. TBG deficiency exhibits clinically euthyroid status and does not require treatment. However, physicians unaware of this condition could misdiagnose TBG deficiency as hypothyroidism [12,34,35], despite this rarely occurs in endocrinologist’s practice. Recognizing TBG deficiency is important because misdiagnosis for hypothyroidism might lead to iatrogenic thyrotoxicosis. When only total thyroid hormones level is low while TSH and free T4 are normal, history taking, checking symptoms of hypothyroidism, physical examination, and most importantly testing serum TBG level, along with thyroid autoantibodies or ultrasonography if necessary would help to avoid misdiagnosis.
This study has some limitations. First, it was conducted in a single medical center with a small number of study subjects. Therefore, it is insufficient to identify the pool of TBG variants in Korea. Second, no investigation of the mutational status of family members of the affected patients was conducted. Additional SERPINA7 gene sequencing in immediate family members of the patient harboring the novel mutation is necessary, to help identify whether the mutation is de novo or inherited. However, a mutation analysis in unrelated subjects might be more representative than in family members, given that TBG variants other than TBG-CDJ had not been identified in Korea before this study.
In conclusion, this study presented four patients diagnosed with TBG deficiency and provided the results of SERPINA7 gene sequencing. As a result, one novel mutation, p.Phe269Cysfs*18, causing TBD-PD and three cases of TBG-CDJ were found. Along with previous mutation analyses in Korea, this result suggests that TBG-CDJ could be a dominant variant in the Korean population. The novel mutation p.Phe269Cysfs*18 might also partially contribute to TBG variants in Korea, but further analyses of family members and other unrelated TBG deficiency patients is required. Early recognition of TBG deficiency in patients with discordant TFTs is necessary to prevent improper treatment. Although SERPINA7 gene sequencing is not necessary to diagnose TBG deficiency, it could provide supplementary information about the TBG variants in Korea.
Supplementary Material
Supplemental Table S1.
Polymerase Chain Reaction Primer Sets for Sanger Sequencing of NM_000354.6 (SERPINA7)
REFERENCES
1. DeGroot LJ. Endocrinology. 2nd ed. Philadelphia: Saunders;1989. Chapter, Transport, cellular uptake, and metabolism of thyroid hormone. p. 541–61.
2. Werner SC, Braverman LE, Ingbar SH. Werner’s the thyroid: a fundamental and clinical text. 5th ed. Philadelphia: Lippincott;1986. Chapter 6, Hormone transport in blood. p. 116–27.
3. Hayashi Y, Mori Y, Janssen OE, Sunthornthepvarakul T, Weiss RE, Takeda K, et al. Human thyroxine-binding globulin gene: complete sequence and transcriptional regulation. Mol Endocrinol. 1993; 7:1049–60.
4. Oppenheimer JH. Role of plasma proteins in the binding, distribution and metabolism of the thyroid hormones. N Engl J Med. 1968; 278:1153–62.

5. Trent JM, Flink IL, Morkin E, van Tuinen P, Ledbetter DH. Localization of the human thyroxine-binding globulin gene to the long arm of the X chromosome (Xq21-22). Am J Hum Genet. 1987; 41:428–35.
6. Janssen OE, Bertenshaw R, Takeda K, Weiss R, Refetoff S. Molecular basis of inherited thyroxine-binding globulin defects. Trends Endocrinol Metab. 1992; 3:49–53.

7. Li P, Janssen OE, Takeda K, Bertenshaw RH, Refetoff S. Complete thyroxine-binding globulin (TBG) deficiency caused by a single nucleotide deletion in the TBG gene. Metabolism. 1991; 40:1231–4.
8. Gomes-Lima CJ, Maciel AA, Andrade MO, Cunha VS, Mazzeu JF, Bleicher L, et al. Thyroxine-binding globulin deficiency due to a novel SERPINA7 mutation: clinical characterization, analysis of X-chromosome inactivation pattern and protein structural modeling. Gene. 2018; 666:58–63.

9. Okamoto H, Mori Y, Tani Y, Nakagomi Y, Sano T, Ohyama K, et al. Molecular analysis of females manifesting thyroxine-binding globulin (TBG) deficiency: selective X-chromosome inactivation responsible for the difference between phenotype and genotype in TBG-deficient females. J Clin Endocrinol Metab. 1996; 81:2204–8.
10. Arisaka O, Hosaka A, Shimura N, Yabuta K. Thyroxinebinding globulin deficiency misdiagnosed as hypothyroidism. J Pediatr. 1993; 123:333–4.

11. Dang PP, Xiao WW, Shan ZY, Xi Y, Wang RR, Yu XH, et al. Novel frameshift mutation causes early termination of the thyroxine-binding globulin protein and complete thyroxinebinding globulin deficiency in a Chinese family: a case report. World J Clin Cases. 2019; 7:3887–94.

12. Soheilipour F, Fazilaty H, Jesmi F, Gahl WA, Behnam B. First report of inherited thyroxine-binding globulin deficiency in Iran caused by a known de novo mutation in SERPINA7. Mol Genet Metab Rep. 2016; 8:13–6.

13. Gawandi S, Jothivel K, Kulkarni S. Identification of a novel mutation in thyroxine-binding globulin (TBG) gene associated with TBG-deficiency and its effect on the thyroid function. J Endocrinol Invest. 2022; 45:731–9.

14. Pappa T, Ferrara AM, Refetoff S. Inherited defects of thyroxine-binding proteins. Best Pract Res Clin Endocrinol Metab. 2015; 29:735–47.
15. Park SJ, Suh JS, Jung MH, Lee HJ, Suh BK, Lee WB, et al. A single nucleotide deletion resulting in frameshift in two Korean neonates with thyroxine-binding globulin deficiency. Korean J Pediatr. 2005; 48:1252–5.
16. Yamamori I, Mori Y, Miura Y, Tani Y, Imamura S, Oiso Y, et al. Gene screening of 23 Japanese families with complete thyroxine-binding globulin deficiency: identification of a nucleotide deletion at codon 352 as a common cause. Endocr J. 1993; 40:563–9.
17. Baek CH, Choi EY, Jang YB, Wee S. Sequence of thyroxin-binding globulin gene in a Korean family with complete TBG deficiency. Korean J Genet. 1996; 18:183–9.
18. Frequency Filter [Internet]. James Ware;2016. [cited 2022 Nov 23]. Available from: http://cardiodb.org/allelefrequencyapp.
19. Whiffin N, Minikel E, Walsh R, O’Donnell-Luria AH, Karczewski K, Ing AY, et al. Using high-resolution variant frequencies to empower clinical genome interpretation. Genet Med. 2017; 19:1151–8.

20. Yamamori I, Mori Y, Seo H, Hirooka Y, Imamura S, Miura Y, et al. Nucleotide deletion resulting in frameshift as a possible cause of complete thyroxine-binding globulin deficiency in six Japanese families. J Clin Endocrinol Metab. 1991; 73:262–7.
21. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015; 17:405–24.

22. Kim DM, Kim HK, Yoo SJ. A case of complete deficiency of total thyroxine-binding globulin (TBG) associated with Graves’ disase. Endocr Abstr. 2009; 20:130.
23. Ihm SH, Yoo JM, Hong SG, Choi MG, Yoo HJ, Park SW, et al. Gene screening of complete thyroxine-binding globulin deficiency by allele specific amplification. Korean J Med. 1995; 49:651–8.
24. Lee JW, Jin JY, Lee J, Lee DH, Hong YH. A study of thyroid function in partial thyroxine-binding globulin deficiency. Soonchunhyang Med Sci. 2015; 21:65–9.

25. Jo IS, Choi HJ, Lee YA, Chung WG, Chang YB. Two cases of congenital TBG deficiency. J Korean Pediatr Soc. 1995; 38:697–701.
26. Hur SM, Kim SH, Kim MJ, Byun DW, Suh KI, Yoo MH, et al. A case of hypothyroxinemia with thyroxine-binding-globulin deficiency. Soonchunhyang Med Sci. 2011; 17:161–3.

27. Lee DC, Lee SH, Yu JH. A case of thyroxine binding globulin deficiency with hypothyroidism. J Korean Pediatr Soc. 2002; 45:796–9.
28. Lee WJ, Lee SY, Lee JE, Kim SY, Lee JH, Lee SJ. Two cases of congenital TBG deficiency: a family study. J Korean Soc Neonatol. 1996; 3:160–3.
29. Refetoff S. Inherited thyroxine-binding globulin abnormalities in man. Endocr Rev. 1989; 10:275–93.

30. Lacka K, Nizankowska T, Ogrodowicz A, Lacki JK. A novel mutation (del 1711 G) in the TBG gene as a cause of complete TBG deficiency. Thyroid. 2007; 17:1143–6.

31. Domingues R, Bugalho MJ, Garrao A, Boavida JM, Sobrinho L. Two novel variants in the thyroxine-binding globulin (TBG) gene behind the diagnosis of TBG deficiency. Eur J Endocrinol. 2002; 146:485–90.

32. Su CC, Wu YC, Chiu CY, Won JG, Jap TS. Two novel mutations in the gene encoding thyroxine-binding globulin (TBG) as a cause of complete TBG deficiency in Taiwan. Clin Endocrinol (Oxf). 2003; 58:409–14.

33. Carvalho GA, Weiss RE, Vladutiu AO, Refetoff S. Complete deficiency of thyroxine-binding globulin (TBG-CD Buffalo) caused by a new nonsense mutation in the thyroxine-binding globulin gene. Thyroid. 1998; 8:161–5.

Fig. 1.
Sanger sequencing chromatogram of the serpin family A member 7 (SERPINA7) variants detected in this study. (A) c.1114del variant was detected in heterozygous patient 1 and 3 and in hemizygous patient 2. (B) A novel hemizygous 2-bp deletion variant, c.806_807del, was detected in patient 4. Electropherograms of a wildtype and the proband are demonstrated. The positions of the deletion are indicated by arrows. The predicted amino acid translation is shown above the sequence. Altered codons in the proband are shown in red.
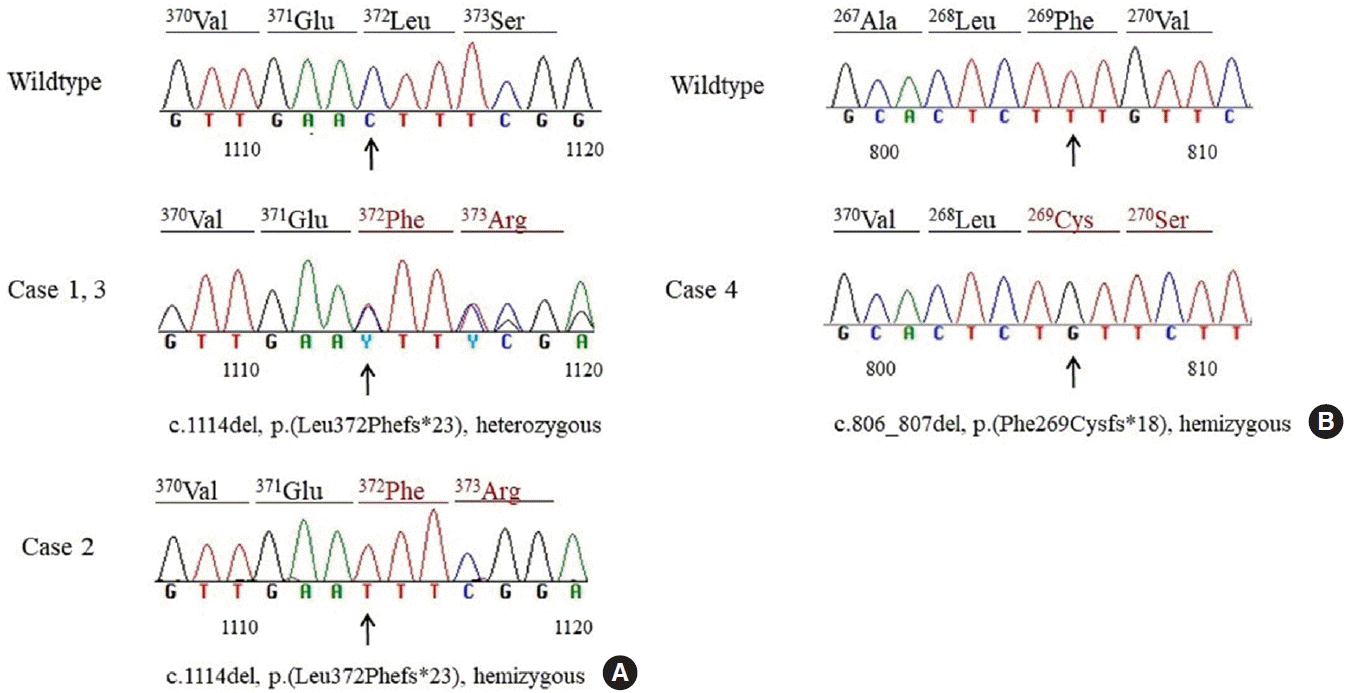
Table 1.
The Results of Baseline Characteristics, Thyroid Function Tests, TBG, Thyroid Autoantibodies, and Results of Direct Sequencing of SERPINA7 Gene
Table 2.
The Detailed Thyroid Hormones and Dosage of Levothyroxine of Patient 1
Table 3.
The Detailed Thyroid Hormones and Dosage of Levothyroxine of Patient 3
Table 4.
Summary of Previous Reports of TBG Deficiency and Mutation Analysis in Korea
| Study | Year | Subject | No. of subjects | Age | Reason for TBG testing | Coexisting thyroid function abnormality | Phenotype | Sequencing | Sequencing in family member (variant) |
|---|---|---|---|---|---|---|---|---|---|
| Jo et al. [25] | 1995 | Neonate | 2 | 4.5 wk | Low T4 on screening | No | TBG-CD | Not done | |
| Lee et al. [28] | 1996 | Neonate | 2 | 1 mo | Low T4 on screening | No | TBG-CD | Not done | |
| Park et al. [15] | 2005 | Neonate | 2 | 3.5 wk | Low T4 on screening | No | TBG-CD | TBG-CDJ | |
| Lee et al. [24] | 2015 | Neonate | 32 | 35.8 day | Low T4 on screening | Hypothyroidism (18/32) | TBG-CD | Not done | |
| Baek et al. [17] | 1996 | Child | 1 | 11 yr | Not specified | No | TBG-CD | TBG-CDJ | Mother (TBG-CDJ) |
| Lee et al. [27] | 2002 | Child | 1 | 22 mo | Low T4 on screening | Hypothyroidism | TBG-CD | Not done | |
| Ihm et al. [23] | 1995 | Adult | 2 | 36, 51 yr | Low T3 and T4 | No | TBG-CD | TBG-CDJ | Two siblings (TBG-CDJ) |
| Kim et al. [22] | 2009 | Adult | 1 | 28 yr | Normal T3, low TSH, high free T4 | Graves’ disease | TBG-CD | TBG-CDJ | |
| Hur et al. [26] | 2011 | Adult | 1 | 68 yr | Low total T3/T4 and high free T4 | No | TBG-CD | Not done |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download