1. Arvan P, Bernal-Mizrachi E, Liu M, Pietropaolo M, Satin L, Schnell S, et al. Molecular aspects of pancreatic beta cell failure and diabetes. Mol Aspects Med. 2015; 42:1–2.

2. Forouhi NG, Koulman A, Sharp SJ, Imamura F, Kroger J, Schulze MB, et al. Differences in the prospective association between individual plasma phospholipid saturated fatty acids and incident type 2 diabetes: the EPIC-InterAct case-cohort study. Lancet Diabetes Endocrinol. 2014; 2:810–8.

3. Lu H, Hao L, Li S, Lin S, Lv L, Chen Y, et al. Elevated circulating stearic acid leads to a major lipotoxic effect on mouse pancreatic beta cells in hyperlipidaemia via a miR-34a-5p-mediated PERK/p53-dependent pathway. Diabetologia. 2016; 59:1247–57.

4. Chu X, Liu L, Na L, Lu H, Li S, Li Y, et al. Sterol regulatory element-binding protein-1c mediates increase of postprandial stearic acid, a potential target for improving insulin resistance, in hyperlipidemia. Diabetes. 2013; 62:561–71.

5. Liu L, Li Y, Guan C, Li K, Wang C, Feng R, et al. Free fatty acid metabolic profile and biomarkers of isolated post-challenge diabetes and type 2 diabetes mellitus based on GCMS and multivariate statistical analysis. J Chromatogr B Analyt Technol Biomed Life Sci. 2010; 878:2817–25.

6. Remedi MS, Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes Obes Metab. 2016; 18(Suppl 1):110–6.

7. Brereton MF, Rohm M, Ashcroft FM. β-Cell dysfunction in diabetes: a crisis of identity? Diabetes Obes Metab. 2016; 18(Suppl 1):102–9.
8. Weir GC, Bonner-Weir S. Five stages of evolving beta-cell dysfunction during progression to diabetes. Diabetes. 2004; 53 Suppl 3:S16–21.

9. Marchetti P, Bugliani M, Lupi R, Marselli L, Masini M, Boggi U, et al. The endoplasmic reticulum in pancreatic beta cells of type 2 diabetes patients. Diabetologia. 2007; 50:2486–94.

10. Kitamura T. The role of FOXO1 in β-cell failure and type 2 diabetes mellitus. Nat Rev Endocrinol. 2013; 9:615–23.

11. Eguchi K, Manabe I, Oishi-Tanaka Y, Ohsugi M, Kono N, Ogata F, et al. Saturated fatty acid and TLR signaling link β cell dysfunction and islet inflammation. Cell Metab. 2012; 15:518–33.

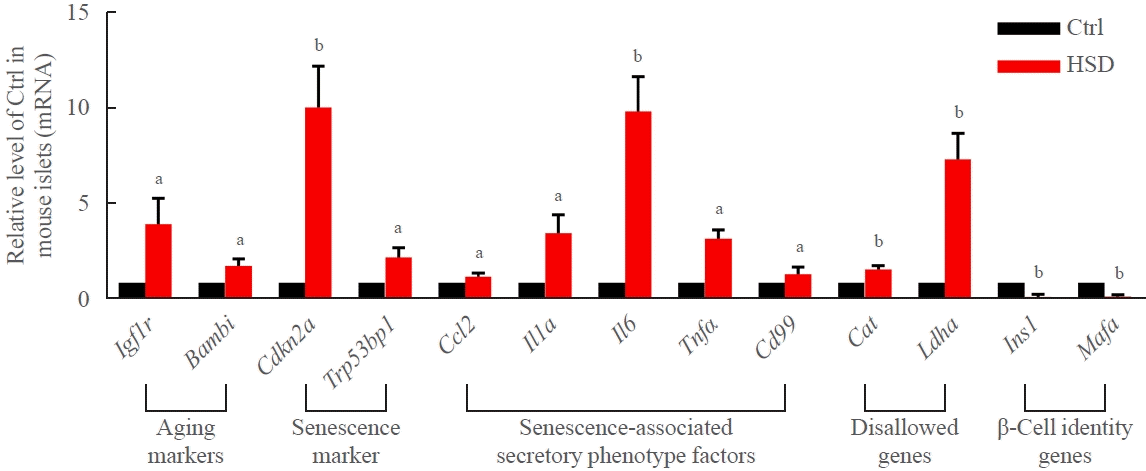
12. Aguayo-Mazzucato C. Functional changes in beta cells during ageing and senescence. Diabetologia. 2020; 63:2022–9.

13. Aguayo-Mazzucato C, Andle J, Lee TB Jr, Midha A, Talemal L, Chipashvili V, et al. Acceleration of β cell aging determines diabetes and senolysis improves disease outcomes. Cell Metab. 2019; 30:129–42.

14. Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br J Pharmacol. 2018; 175:3190–9.

15. Reiter RJ, Mayo JC, Tan DX, Sainz RM, Alatorre-Jimenez M, Qin L. Melatonin as an antioxidant: under promises but over delivers. J Pineal Res. 2016; 61:253–78.

16. Tamura H, Kawamoto M, Sato S, Tamura I, Maekawa R, Taketani T, et al. Long-term melatonin treatment delays ovarian aging. J Pineal Res. 2017; 62:e12381.

17. Carbajo-Pescador S, Ordonez R, Benet M, Jover R, GarciaPalomo A, Mauriz JL, et al. Inhibition of VEGF expression through blockade of Hif1α and STAT3 signalling mediates the anti-angiogenic effect of melatonin in HepG2 liver cancer cells. Br J Cancer. 2013; 109:83–91.

18. Xia Y, Chen S, Zeng S, Zhao Y, Zhu C, Deng B, et al. Melatonin in macrophage biology: current understanding and future perspectives. J Pineal Res. 2019; 66:e12547.

19. Garaulet M, Qian J, Florez JC, Arendt J, Saxena R, Scheer FA. Melatonin effects on glucose metabolism: time to unlock the controversy. Trends Endocrinol Metab. 2020; 31:192–204.

20. Li X, Zhang M, Tang W. Effects of melatonin on streptozotocin-induced retina neuronal apoptosis in high blood glucose rat. Neurochem Res. 2013; 38:669–76.

21. Costes S, Boss M, Thomas AP, Matveyenko AV. Activation of melatonin signaling promotes β-cell survival and function. Mol Endocrinol. 2015; 29:682–92.

22. Lee YH, Jung HS, Kwon MJ, Jang JE, Kim TN, Lee SH, et al. Melatonin protects INS-1 pancreatic β-cells from apoptosis and senescence induced by glucotoxicity and glucolipotoxicity. Islets. 2020; 12:87–98.

23. He H, Dong W, Huang F. Anti-amyloidogenic and antiapoptotic role of melatonin in Alzheimer disease. Curr Neuropharmacol. 2010; 8:211–7.

24. Park JH, Shim HM, Na AY, Bae KC, Bae JH, Im SS, et al. Melatonin prevents pancreatic β-cell loss due to glucotoxicity: the relationship between oxidative stress and endoplasmic reticulum stress. J Pineal Res. 2014; 56:143–53.
25. Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011; 12:861–74.

26. Flynt AS, Lai EC. Biological principles of microRNA-mediated regulation: shared themes amid diversity. Nat Rev Genet. 2008; 9:831–42.

27. Martinez-Sanchez A, Rutter GA, Latreille M. MiRNAs in β-cell development, identity, and disease. Front Genet. 2017; 7:226.

28. Yu Y, Guo R, Zhang Y, Shi H, Sun H, Chu X, et al. miRNAmRNA profile and regulatory network in stearic acid-treated β-cell dysfunction. J Endocrinol. 2020; 246:13–27.

29. Goldstein JL, Basu SK, Brown MS. Receptor-mediated endocytosis of low-density lipoprotein in cultured cells. Methods Enzymol. 1983; 98:241–60.
30. Kim HS, Han TY, Yoo YM. Melatonin-mediated intracellular insulin during 2-deoxy-d-glucose treatment is reduced through autophagy and EDC3 protein in insulinoma INS-1E cells. Oxid Med Cell Longev. 2016; 2016:2594703.

31. Guo F, Huang C, Liao X, Wang Y, He Y, Feng R, et al. Beneficial effects of mangiferin on hyperlipidemia in high-fatfed hamsters. Mol Nutr Food Res. 2011; 55:1809–18.

32. Guo R, Yu Y, Zhang Y, Li Y, Chu X, Lu H, et al. Overexpression of miR-297b-5p protects against stearic acid-induced pancreatic β-cell apoptosis by targeting LATS2. Am J Physiol Endocrinol Metab. 2020; 318:E430–9.

33. Zhu MJ, Liu BY, Shi L, Wang X, Wang Y. mTOR-autophagy promotes pulmonary senescence through IMP1 in chronic toxicity of methamphetamine. J Cell Mol Med. 2020; 24:12082–93.

34. Lee BY, Han JA, Im JS, Morrone A, Johung K, Goodwin EC, et al. Senescence-associated beta-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006; 5:187–95.
35. Shi H, Bressan R. RNA extraction. Methods Mol Biol. 2006; 323:345–8.

36. Fleischer N, Chen C, Surana M, Leiser M, Rossetti L, Pralong W, et al. Functional analysis of a conditionally transformed pancreatic beta-cell line. Diabetes. 1998; 47:1419–25.

37. Knaack D, Fiore DM, Surana M, Leiser M, Laurance M, Fusco-DeMane D, et al. Clonal insulinoma cell line that stably maintains correct glucose responsiveness. Diabetes. 1994; 43:1413–7.

38. Fan X, Gu S, Lei J, Gu S, Yang L. Controlled release of insulin based on temperature and glucose dual responsive biomicrocapsules. Molecules. 2022; 27:1686.

39. Mziaut H, Henniger G, Ganss K, Hempel S, Wolk S, McChord J, et al. MiR-132 controls pancreatic beta cell proliferation and survival through Pten/Akt/Foxo3 signaling. Mol Metab. 2020; 31:150–62.

40. Giuliani A, Prattichizzo F, Micolucci L, Ceriello A, Procopio AD, Rippo MR. Mitochondrial (Dys) function in inflammaging: do MitomiRs influence the energetic, oxidative, and inflammatory status of senescent cells? Mediators Inflamm. 2017; 2017:2309034.

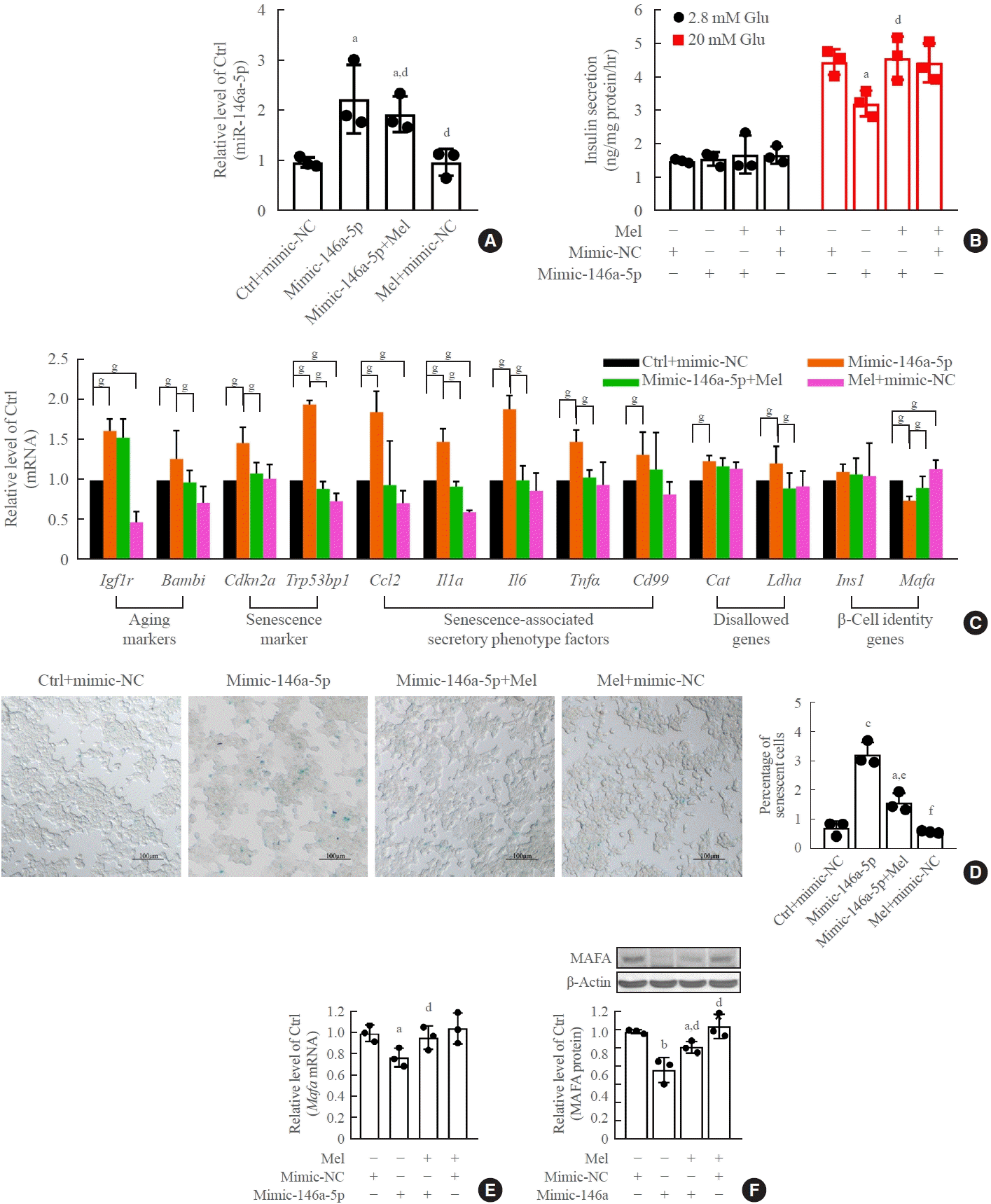
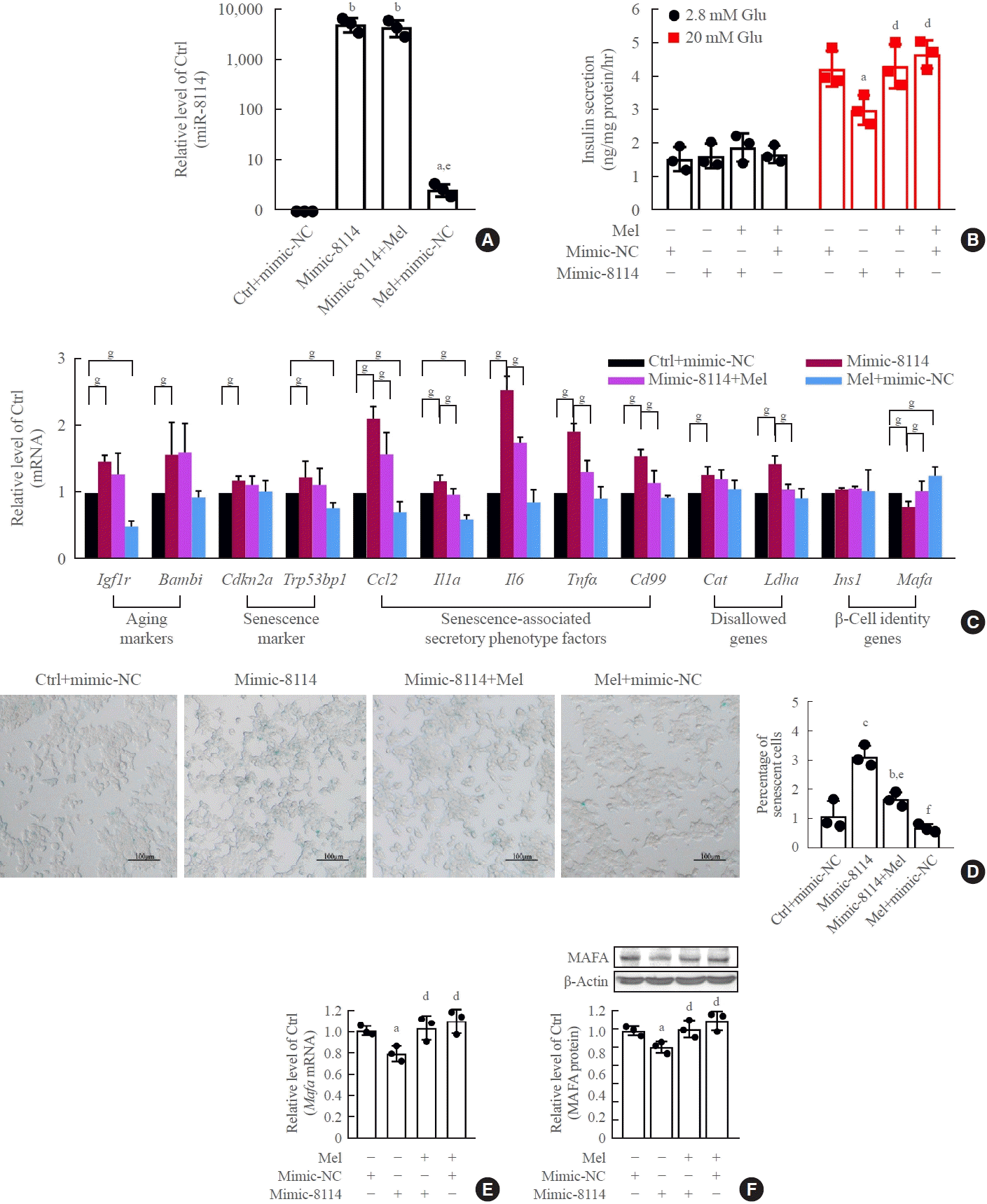
41. Iacona JR, Lutz CS. miR-146a-5p: expression, regulation, and functions in cancer. Wiley Interdiscip Rev RNA. 2019; 10:e1533.

42. Olivieri F, Prattichizzo F, Giuliani A, Matacchione G, Rippo MR, Sabbatinelli J, et al. miR-21 and miR-146a: the microRNAs of inflammaging and age-related diseases. Ageing Res Rev. 2021; 70:101374.

43. Lo WY, Wang SJ, Wang HJ. Non-canonical interaction between O-linked N-acetylglucosamine transferase and miR146a-5p aggravates high glucose-induced endothelial inflammation. Front Physiol. 2020; 11:1091.

44. Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, et al. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008; 57:2728–36.
45. Roggli E, Britan A, Gattesco S, Lin-Marq N, Abderrahmani A, Meda P, et al. Involvement of microRNAs in the cytotoxic effects exerted by proinflammatory cytokines on pancreatic beta-cells. Diabetes. 2010; 59:978–86.
46. Ebrahimi AG, Hollister-Lock J, Sullivan BA, Tsuchida R, Bonner-Weir S, Weir GC. Beta cell identity changes with mild hyperglycemia: implications for function, growth, and vulnerability. Mol Metab. 2020; 35:100959.

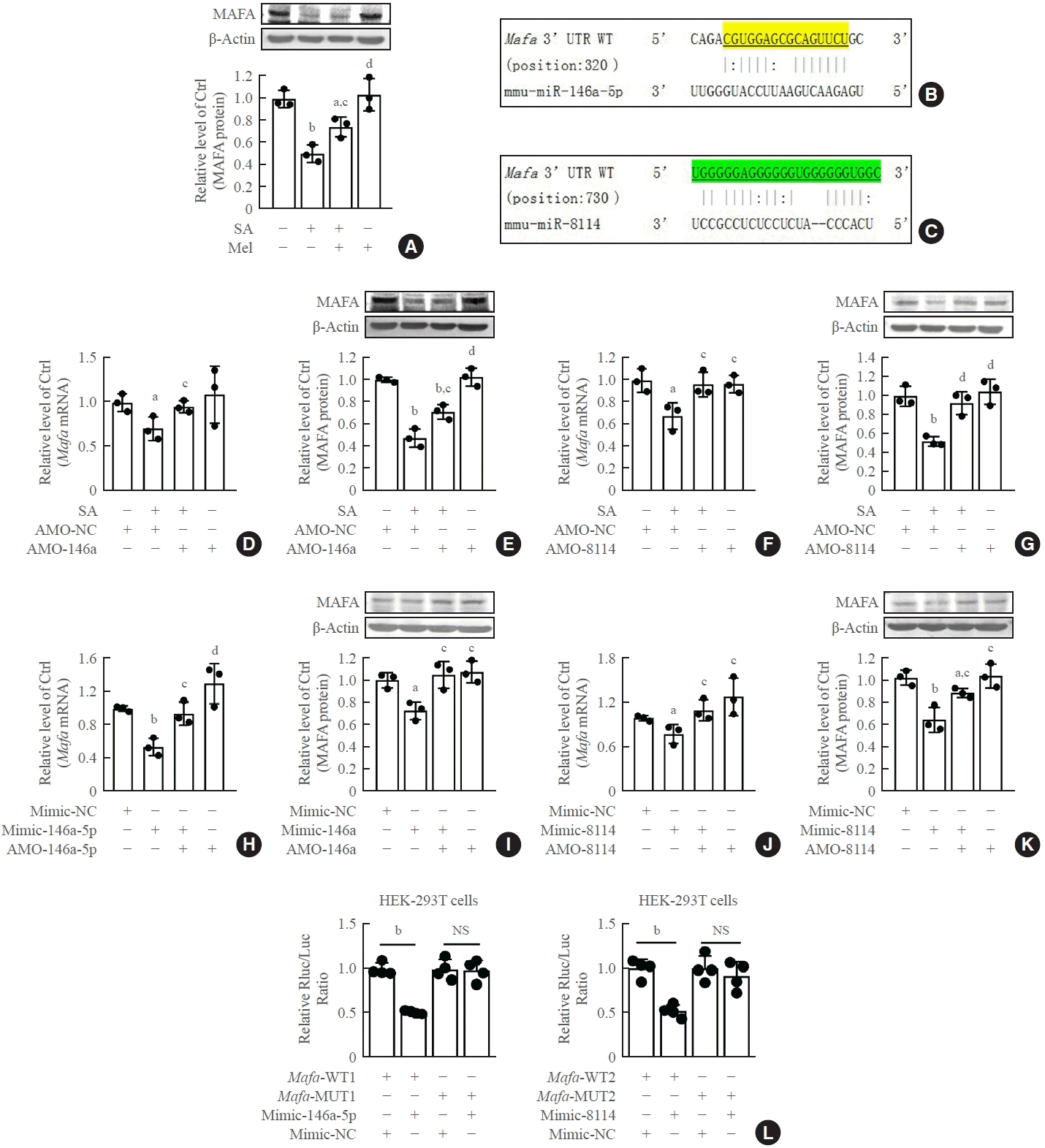
47. Hang Y, Stein R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metab. 2011; 22:364–73.

48. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015; 64:2289–98.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download