INTRODUCTION
METHODS
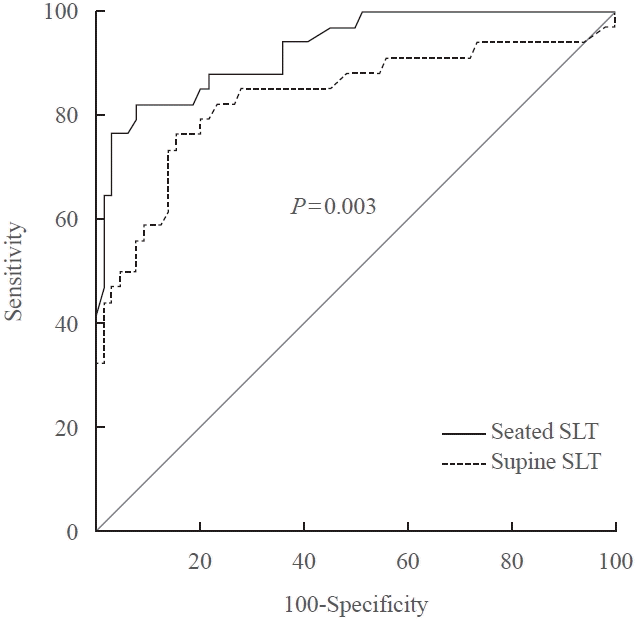
RESULTS
Table 1.
| Characteristic | PA-confirmed (n=34) | PA-excluded (n=64) | P value | |
|---|---|---|---|---|
| Age, yr | 56±13 | 58±11 | 0.524 | |
| Male sex | 24 (70.6) | 28 (43.8) | 0.010 | |
| BMI, kg/m2 | 25.9±3.6 | 25.2±2.9 | 0.324 | |
| SBP, mm Hg | 146±22 | 139±22 | 0.136 | |
| DBP, mm Hg | 83±14 | 81±14 | 0.465 | |
| Potassium, mmol/L | 3.9±0.4 | 4.2±0.6 | 0.036 | |
| Screening test–PAC/PRA/ARR | ||||
| PAC by LC-MS/MS, ng/dL | 15.6 (8.8–29.1) | 6.35 (4.4–12.1) | <0.001 | |
| PAC by RIA, ng/dL | 18.6 (11.1–34.7)a | 8.9 (6.2–13.3)b | <0.001 | |
| PRA by LC-MS/MS, ng/mL/hr | 0.12 (0.07–0.26) | 0.14 (0.08–0.28) | 0.566 | |
| PRA by RIA, ng/mL/hr | 0.10 (0.10–0.20) | 0.20 (0.10–0.25) | 0.944 | |
| ARR by LC-MS/MS, ng/dL per ng/mL/hr | 101.9 (55.2–323.9) | 50.0 (20.8–86.5) | 0.005 | |
| ARR by RIA, ng/dL per ng/mL/hr | 116.2 (59.5–273.1) | 59.6 (38.3–85.5) | 0.001 | |
| Supine SLT | ||||
| Post-SLT PAC by RIA, ng/dL | 10.7 (7.1–34.0) | 3.6 (2.7–5.1) | <0.001 | |
| Seated SLT | ||||
| Post-SLT PAC by LC-MS/MS, ng/dL | 9.7 (7.9–20.0) | 2.9 (1.9–3.7) | <0.001 | |
| Post-SLT PAC by RIA, ng/dL | 11.1 (7.5–15.3) | 4.0 (2.5–4.7) | <0.001 | |
Values are expressed as mean±standard deviation, number (%), or median (interquartile range). Categorical variables are shown as frequency. P value was calculated using an independent or paired t test for continuous variables and the chi-square test for categorical variables. PA was confirmed or excluded by seated SLT; if the post-SLT PAC measured by LC-MS/MS was greater than 5.8 ng/dL (162 pmol/L), PA was confirmed.
SLT, saline loading test; PA, primary aldosteronism; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; PAC, plasma aldosterone concentration; PRA, plasma renin activity; ARR, aldosterone/renin ratio; LC-MS/MS, liquid chromatography-tandem mass spectrometry; RIA, radioimmunoassay.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download