INTRODUCTION
METHODS
Materials
Cell culture
Western blot analysis
Cell proliferation assay
Measurement of reactive oxygen species
Transmission electron microscopy
Animal experiments
Hematoxylin and eosin stain and Masson’s trichrome stain
Hepatic malondialdehyde determination
Quantitative real time-polymerase chain reaction
Statistical analyses
RESULTS
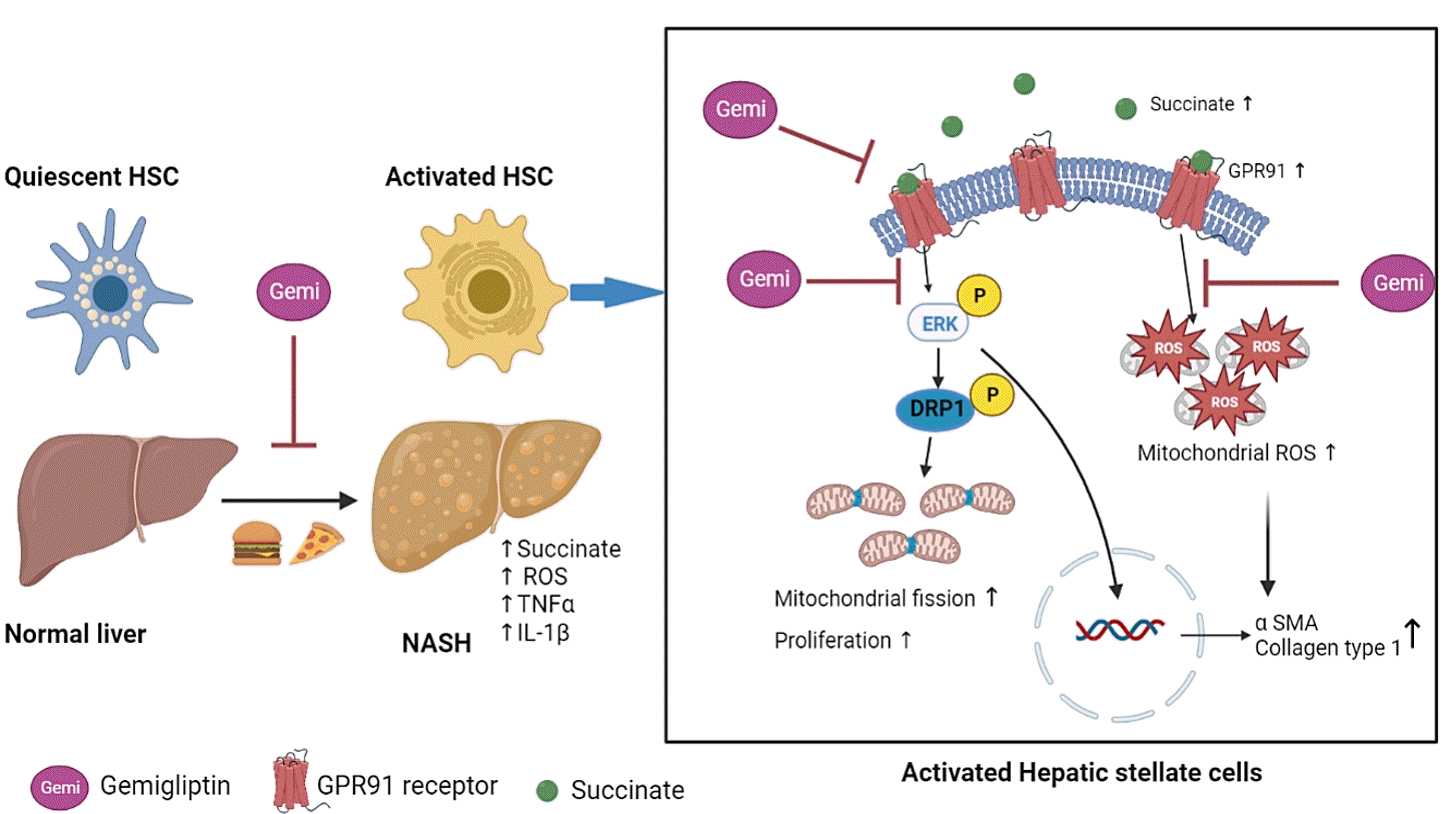
Gemigliptin ameliorated HSC activation
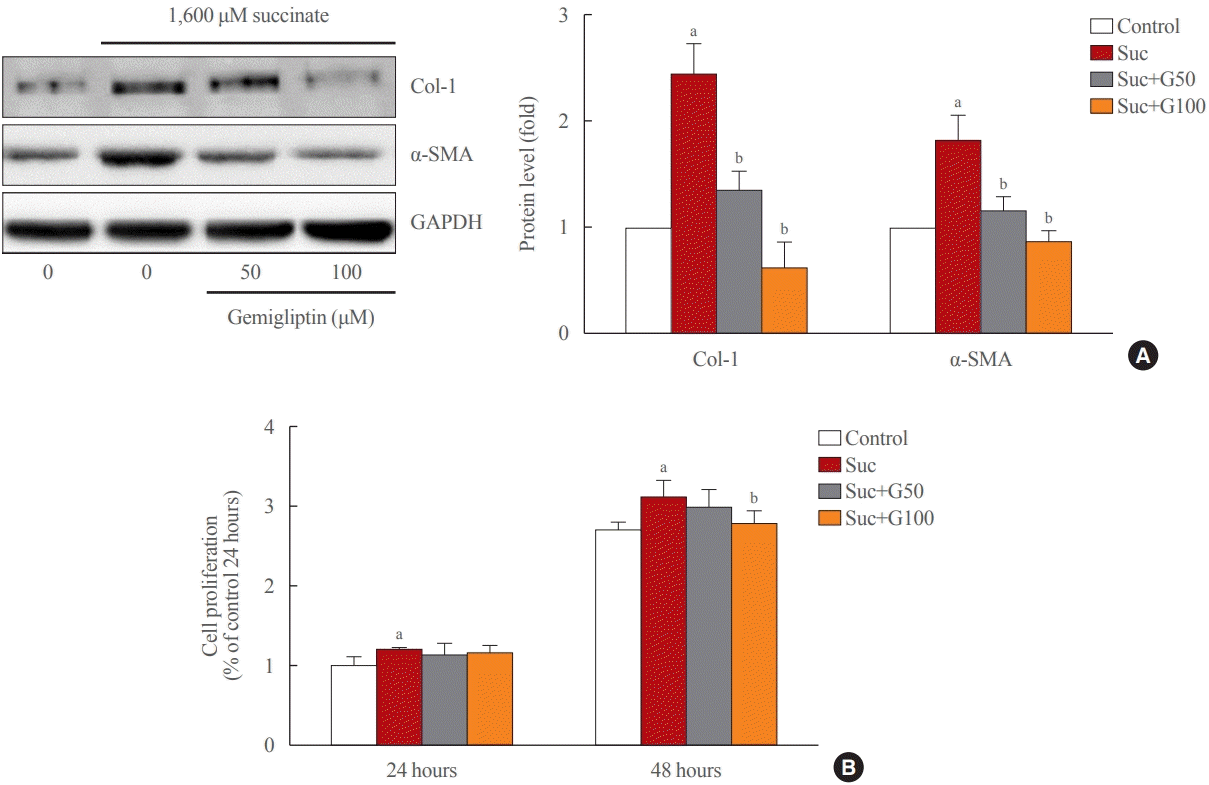 | Fig. 1.Gemigliptin ameliorated succinate-induced hepatic stellate cell activation and proliferation. (A) LX-2 cells were treated with 1,600 μM succinate and co-treated with 50 or 100 μM gemigliptin for 24 hours. Western blot analysis of α-smooth muscle actin (α-SMA) and collagen type 1 (Col-1) expression in LX-2 cells. The band intensities in Western blot images were quantified using Image Lab and plotted to the right of the representative blot images. The protein levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. (B) The proliferation of LX-2 cells that were treated with 1,600 μM succinate and co-treated with 50 or 100 μM gemigliptin for 24 and 48 hours. aP<0.05 vs. control; bP<0.05 vs. succinate (mean±standard error of the mean, n=3). |
Gemigliptin inhibited the succinate-GPR91 signaling pathway in HSCs
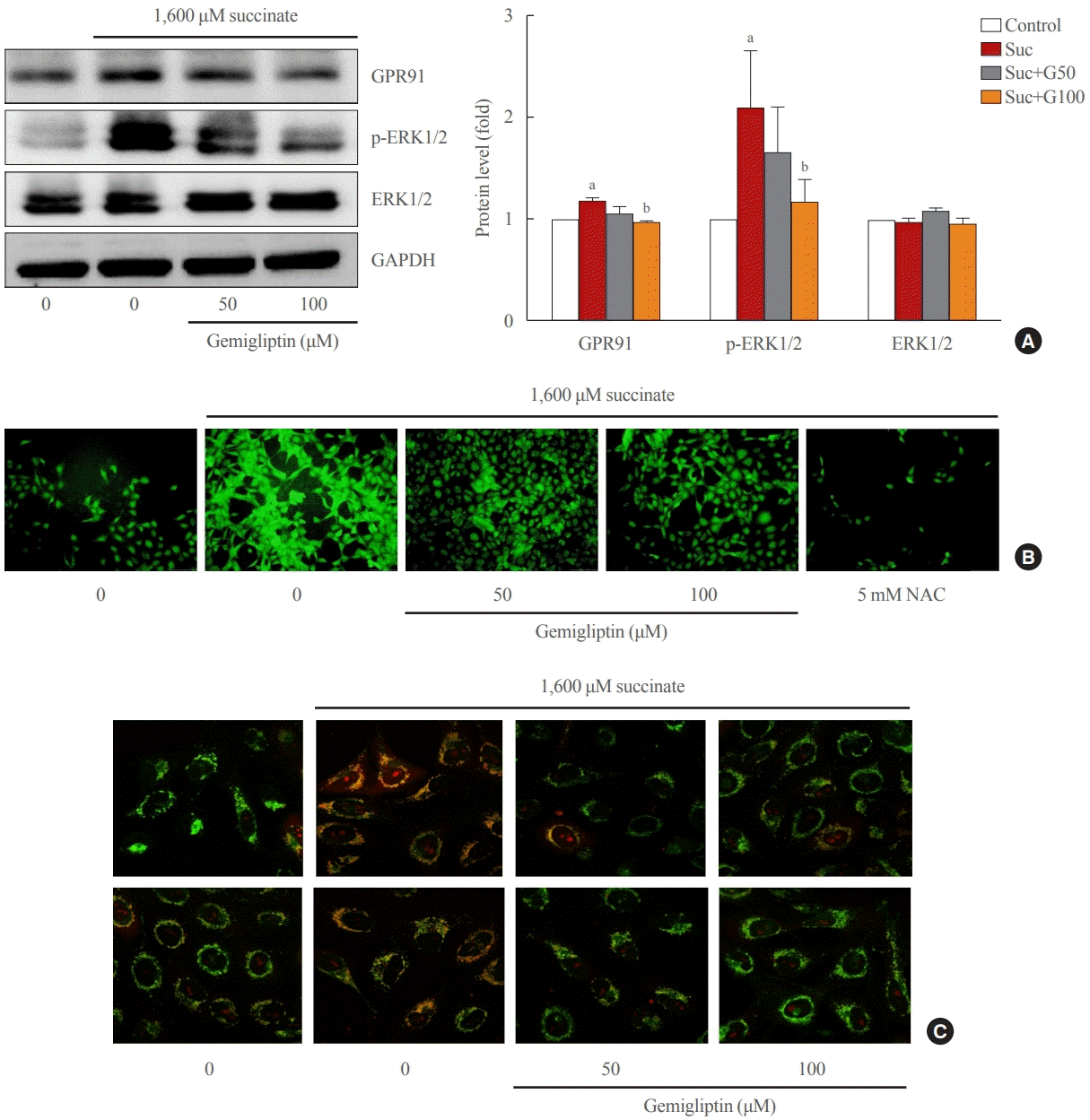 | Fig. 2.Gemigliptin inhibited the succinate-G-protein coupled receptor 91 (GPR91) signaling pathway and reactive oxygen species (ROS) production in hepatic stellate cells. LX-2 cells were treated with 1,600 μM succinate and co-treated with 50 or 100 μM gemigliptin for 24 hours. (A) Western blot analysis of GPR91, phospho-extracellular signal-regulated kinase 1/2 (p-ERK1/2), and total ERK1/2 expression in LX-2 cells. The band intensities in Western blot images were quantified using Image Lab and plotted to the right of the representative blot images. The protein levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. (B) Determination of cellular ROS by the dichlorofluorescin diacetate (DCFDA) assay, fluorescence microscopic images of treated cells. (C) Mitochondrial localization of ROS in LX-2 cells in various treatment groups imaged using confocal scanning microscopy (green: mitochondria; red: MitoSOX). NAC, N-acetyl-L-cysteine. aP<0.05 vs. control; bP<0.05 vs. succinate (mean±standard error of the mean, n=3). |
Gemigliptin alleviated succinate-induced mitochondrial dysfunction
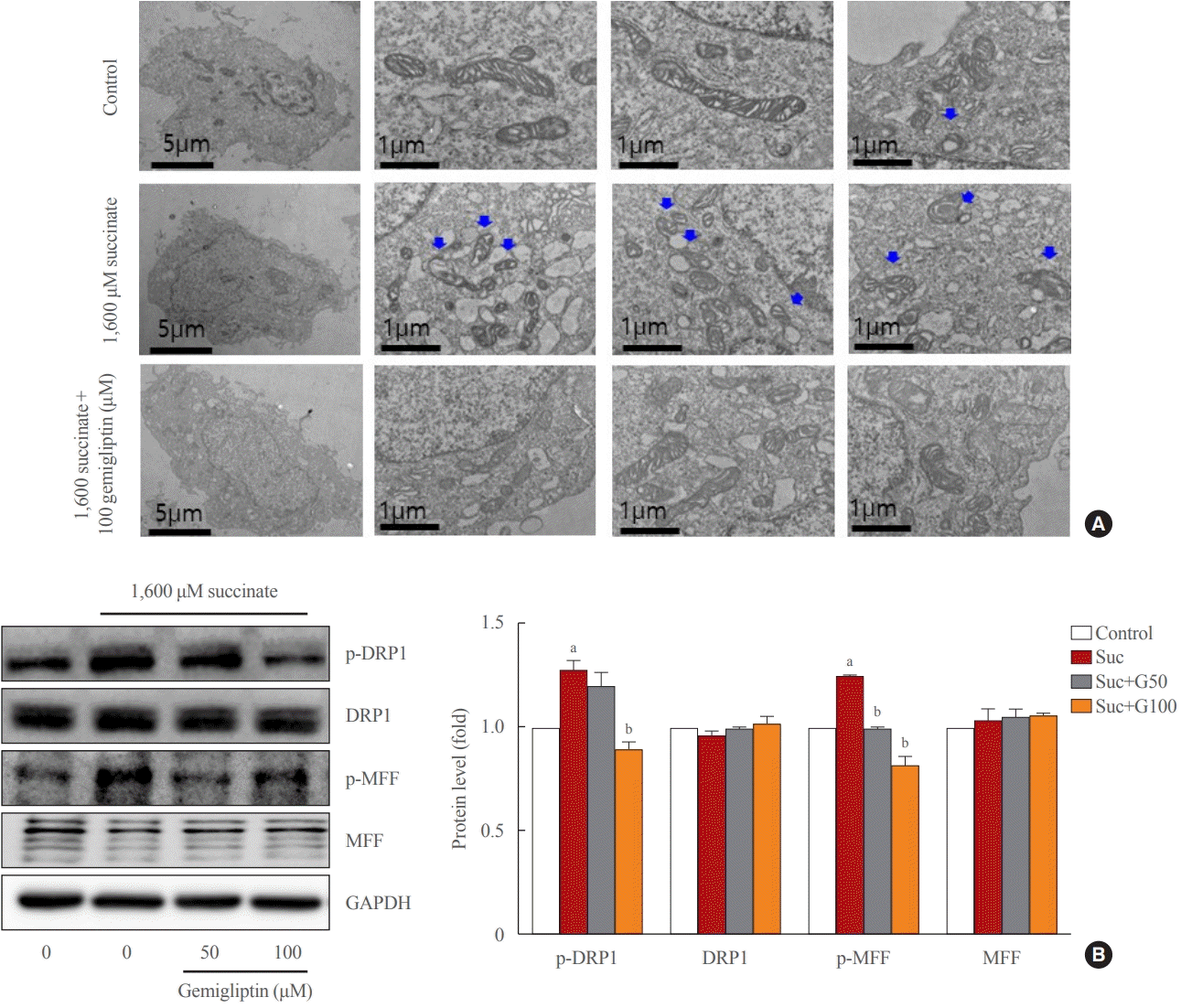 | Fig. 3.Gemigliptin alleviated succinate-induced mitochondrial fragmentation. LX-2 cells were treated with 1,600 μM succinate and cotreated with 50 or 100 μM gemigliptin for 1 hour. (A) Mitochondrial fission was detected using transmission electron microscopy. The first left column shows a single LX-2 cell in the control and treatment groups (scale bar, 2 μm). The two panels in the right column are high-magnification images (scale bar, 1 μm). Blue arrows indicate mitochondria. (B) Western blot analysis of phospho-dynamin-related protein 1 (p-DRP1), phospho-mitochondrial fission factor (p-MFF), total DRP1, and total MFF expression in LX-2 cells. The band intensities in Western blot images were quantified using Image Lab and plotted to the right of the representative blot images. The protein levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) expression. aP<0.05 vs. control; bP<0.05 vs. succinate (mean±standard error of the mean, n=3). |
Gemigliptin attenuated liver inflammation and fibrosis
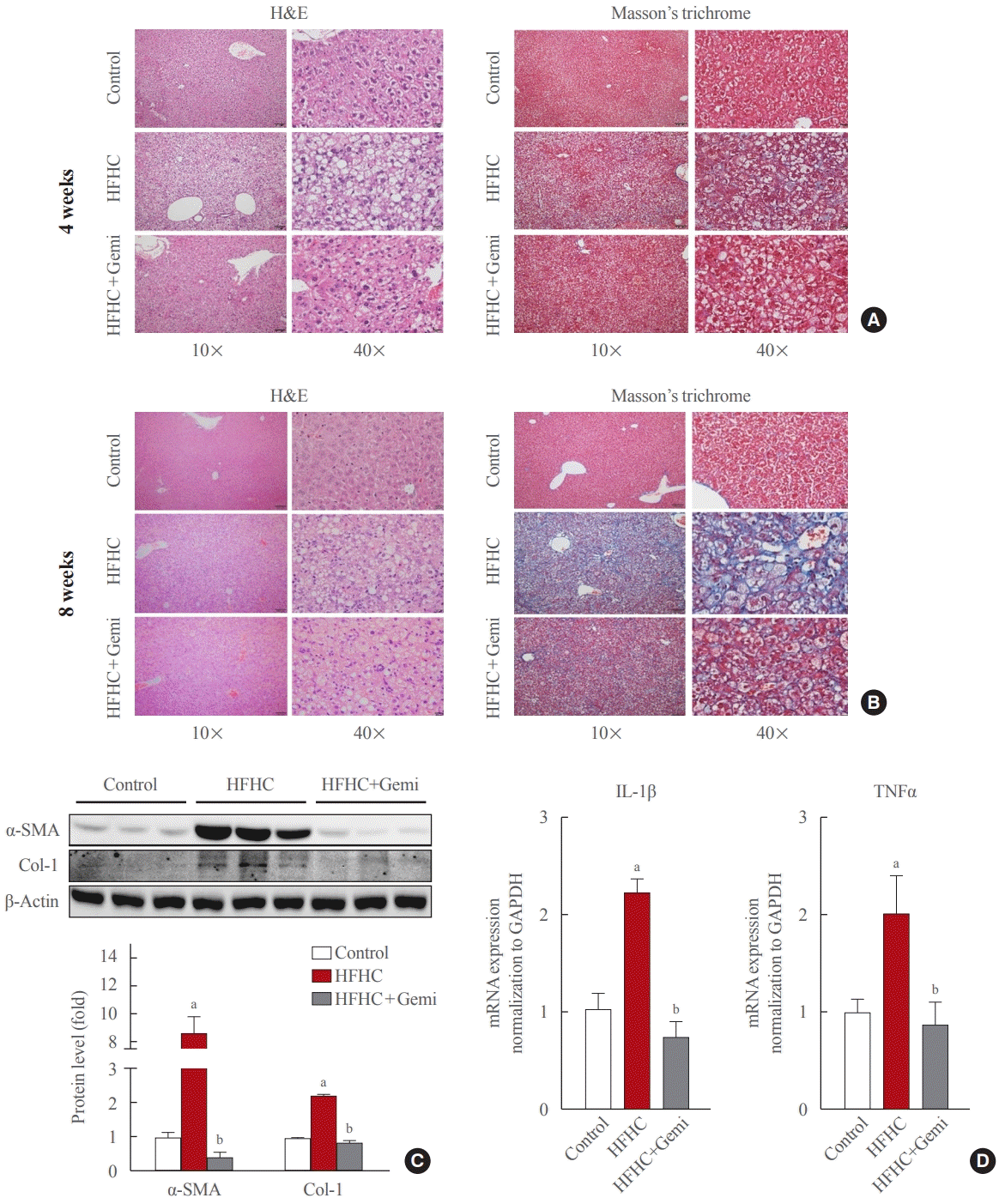 | Fig. 4.Gemigliptin attenuated liver fibrosis and inflammation in a high-fat, high-cholesterol diet mouse model. (A) H&E (left panels) and Masson’s trichrome staining (right panels) were performed to evaluate steatosis and liver fibrosis, at 4 weeks of feeding, respectively. The magnification of the image is shown. (B) H&E (left panels) and Masson’s trichrome staining (right panels) were performed to evaluate steatosis and liver fibrosis after 8 weeks of feeding, respectively. The magnification of the image is shown. (C) Western blot analysis of α-smooth muscle actin (α-SMA), and collagen type 1 (Col-1) expression in the liver from control, high-fat, high-cholesterol (HFHC) dietfed, and HFHC diet+gemigliptin-treated mice. Representative blots are shown above of the plots of the corresponding band intensities. (D) Interleukin-1β (IL-1β) and tumor necrosis factor-α (TNFα) mRNA expression levels in liver tissue were measured in the liver from control, HFHC diet-fed, and HFHC diet+gemigliptin-treated mice. aP<0.05 vs. control group; bP<0.05 vs. HFHC diet group (mean±standard error of the mean; control group [n=5], HFHC group [n=11], HFHC+gemigliptin group [n=11]). |
Gemigliptin protected the liver from oxidative stress and mitochondrial fission
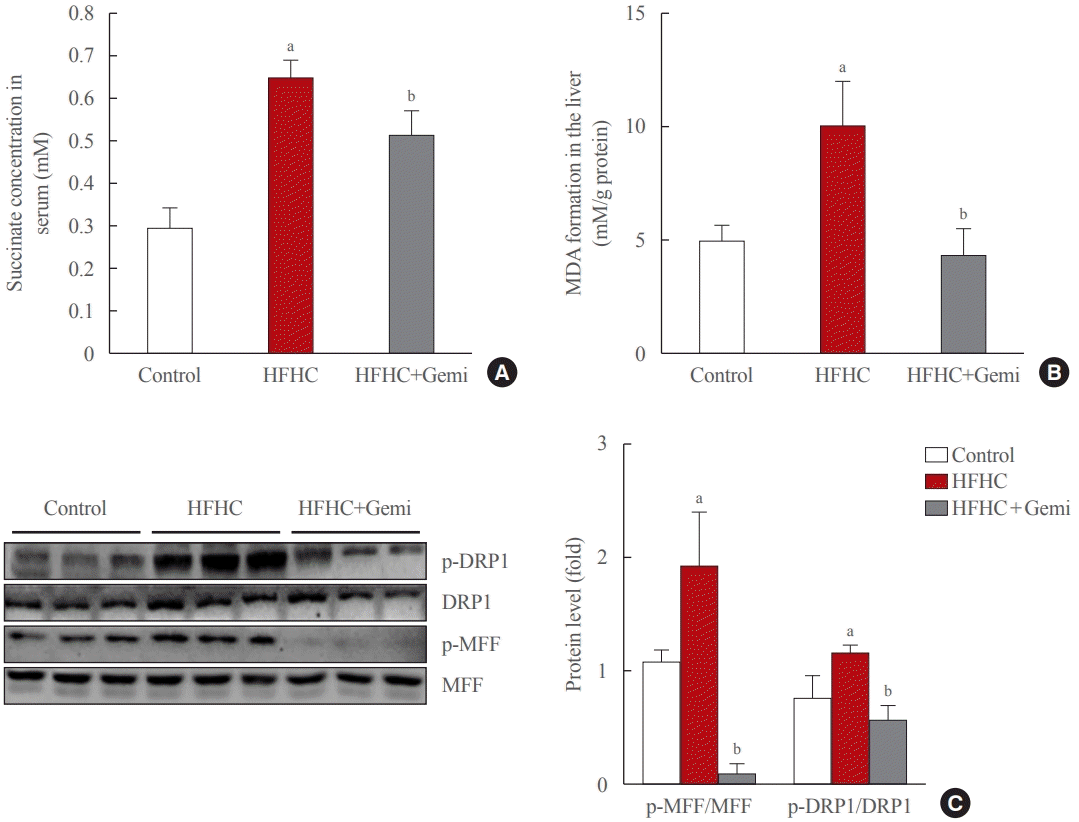 | Fig. 5.Gemigliptin protected the liver from oxidative stress and mitochondrial fission. (A) Succinate concentration in mouse serum samples collected from the indicated groups. (B) malondialdehyde (MDA) formation was measured in the liver from control, high-fat high-cholesterol (HFHC) diet-fed, and HFHC diet+gemigliptin-treated mice. (C) Western blot analysis of phospho-dynamin-related protein 1 (p- DRP1), phospho-mitochondrial fission factor (p-MFF), total DRP1, and total MFF expression in the liver from control, HFHC diet-fed, and HFHC diet+gemigliptin-treated mice. Representative blots are shown to the left of the plots of the corresponding band intensities. aP<0.05 vs. control group; bP<0.05 vs. HFHC diet group (mean±standard error of the mean; control group [n=5], HFHC group [n=11], HFHC+ gemigliptin group [n=11]). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download