Abstract
Purpose
The safety, efficiency, and versatility of novel surgical energy devices have been proved by recent studies. This study aims to investigate the impact of surgical energy devices on operative and oncologic outcomes of minimally invasive colorectal cancer surgery.
Methods
The study group included 80 patients who underwent minimally invasive colorectal cancer surgery with a conventional monopolar device and 217 patients with advanced surgical energy devices between August 2015 and December 2017. The propensity score matching for tumor lesion, preoperative level of CEA, and operation technique produced 63 matched pairs.
Results
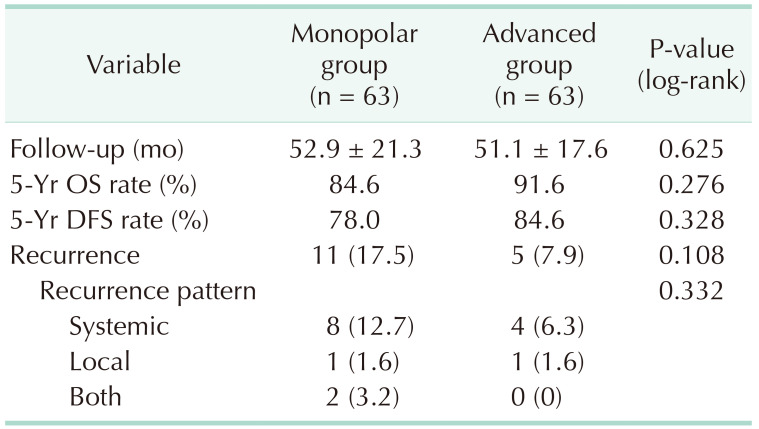
In patient characteristics, there was no significant difference between the groups after the propensity score matching. The amount of blood loss (72 mL vs. 54 mL, P = 0.123) and conversion cases to another surgery (11.1% vs. 4.8%, P = 0.187) tended to be higher in monopolar group, while operation time and intraoperative complications were not significantly different. The short-term clinical outcomes including time to soft diet, the length of hospital stays, and the morbidity within 30 days after surgery or pathologic outcomes were comparable between the groups. During the median follow-up of 52.9 and 51.1 months in each study group, the 5-year overall survival rates of the monopolar and advanced energy groups were 84.6% and 91.6% (P = 0.276), and the 5-year disease-free survival rates were 78.0% and 84.6% (P = 0.328), respectively.
Laparoscopic surgery has gained increasing acceptance for colorectal cancer (CRC) treatment beyond clinical trials since the first report of this minimally invasive surgery (MIS) in 1991 [1]. Robotic surgical systems in MIS were developed recently, in part to overcome several inherent limitations of laparoscopic surgery [23]. MIS for resection of CRC is as effective as open surgery with no negative effect on the overall (OS) and disease-free survival (DFS) rate of patients [4]. Furthermore, this procedure is associated with lower mortality, lower complication rates, and a shorter median length of hospital stay than open approach [56].
In gastrointestinal oncologic surgery, lymphovascular dissection around the major feeding vessels is one of the most important parts. However, it is not easy to safely perform oncologic radical surgery without complications such as massive bleeding [7]. Since Dr. Bovie developed electrosurgery in the 1920s, conventional monopolar electrosurgery devices have been widely used for mesentery dissection and vessel control in MIS of CRC [8]. However, it has several shortcomings including the risk of collateral thermal injury, difficult hemostasis, intraperitoneal temperature variations, smoke production, and necessitating the use of additional tools such as bipolar graspers, sutures, and clips [9]. Several surgical energy devices have been developed in order to overcome these problems, including the electrothermal bipolar vessel sealer, ultrasonic coagulating shears, and devices that integrate both ultrasonic and advanced bipolar energy. These devices revolutionized laparoscopic surgery, allowing for rapid dissection and reliable hemostasis, leading to the ability to perform more complex procedures [10]. Because of various advantages, the use of advanced energy devices is gradually increasing and being applied to CRC surgery [91112]. Some previous studies have proved the safety, efficiency, and versatility of advanced surgical energy devices, having superiority for short-term outcomes such as the amount of blood loss and operation time in CRC surgery to conventional monopolar devices [81113].
Recent studies on energy devices have focused on short-term surgical and short-term clinical outcomes. However, there is no study on the long-term oncologic outcomes of MIS according to types of surgical energy devices. We hypothesized that energy devices may affect the oncologic outcome because they have more tumoricidal and sealing effects around lymphovascular chains than conventional monopolar devices. The purpose of this study was to investigate the impact of surgical energy devices on both operative and oncologic outcomes of MIS for CRC.
The study was approved by the Institutional Review Board of Keimyung University Dongsan Medical Center (No. DSMC 2022-05-083). The requirement for informed consent was waived because of the retrospective nature of the study.
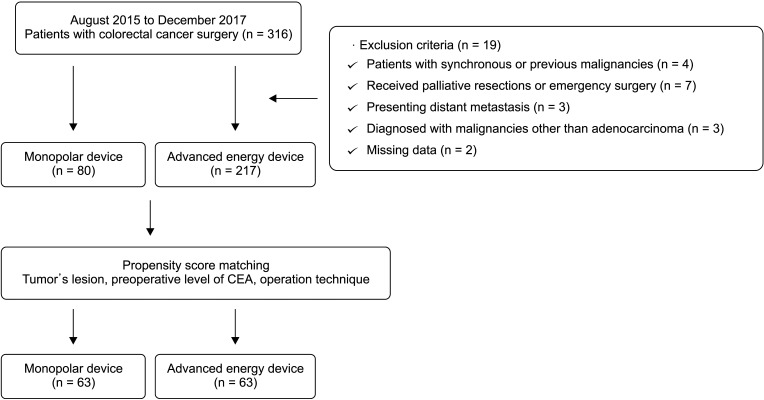
Between August 2015 and December 2017, a total of 316 patients underwent laparoscopic or robotic CRC surgery at Keimyung University Dongsan Medical Center. Exclusion criteria were patients with synchronous or previous malignancies (n = 4), received palliative resections or emergency surgery (n = 7), presenting distant metastasis (n = 3), diagnosed with malignancies other than adenocarcinoma (n = 3), and patients having missing data (n = 2). The study groups included 80 patients who underwent surgery using a conventional monopolar device and 217 patients using an advanced energy device. To minimize the influence of covariates affecting the outcomes, propensity score matching was performed, produced 63 pairs in each group, and a total of 126 patients were finally enrolled in this study (Fig. 1). Information regarding patient demographics, perioperative outcomes, postoperative outcomes, pathologic outcomes, and oncologic outcomes was obtained from a prospectively collected data.
All of the patients underwent a colonoscopy, biopsy, and staging imaging studies including computed tomography of the chest, abdomen, and pelvis, and MRI of the pelvis. In addition, positron emission tomography scans were carried out in select patients. Following the original description [14], we applied the general principles of complete mesocolic or mesorectal excision and central vascular ligation for CRC. The primary tumor was resected by sharp dissection of the visceral plane from the parietal fascia layer along with the entire regional mesocolon in an intact package. For right-sided colon cancer, radical lymphadenectomy was performed along the primary feeding vessels following a vertical line to expose the superior mesenteric vein. For left-sided colon or rectal cancer, high or selective ligation of the inferior mesenteric artery along with lymph node dissection was performed based on the tumor location. The postoperative follow-up and adjuvant treatment were performed according to National Comprehensive Cancer Network guidelines and individual indication.
In this study, we divided the patients into the monopolar energy group and advanced energy group according to the type of energy device used during lymph node dissection and vessel control. The conventional monopolar electrosurgery device was used in the monopolar energy group. Ultrasonic shears (Harmonic ACE, Ethicon Endo-Surgery, Cincinnati, OH, USA) or integrated bipolar and ultrasonic device (Thunderbeat, Olympus Medical Systems Corp., Tokyo, Japan) was used in the advanced energy group.
The tumors located in ascending or transverse colon were classified as right-sided tumors, and the tumors located in descending, sigmoid or rectum were classified as left-sided tumors. Conversion was defined if the surgical technique was interrupted during surgery; MIS techniques to open approach. Surgical complications were classified by the Clavien-Dindo classification, and if a patient had multiple surgical complications, the most severe of them was counted as morbidity.
Tumor stages were classified according to the American Joint Committee on Cancer staging system, 7th edition. OS time was defined as the time between the date of surgery and the date of death or last follow-up visit, and DFS time was defined as the time elapsed between the date of surgery and tumor progression. Patients who died from other causes or were alive without progression or recurrence at the most recent follow-up were treated as censored in the analysis of DFS time. Recurrence was defined as the presence of a radiologically- and/or histologically-confirmed tumor, and the location of recurrence was defined as the first site of recurrence after complete resection. If cancer recurrence occurred in the surgical field, it was defined as local recurrence. Conversely, if cancer recurred outside the surgical field, it was defined as systemic recurrence.
Statistical analyses were performed using IBM SPSS Statistics ver. 25 (IBM Corp., Armonk, NY, USA) and SAS software ver. 9.4 (SAS Institute, Cary, NC, USA). Categorical variables were analyzed using the chi-square or Fisher exact test, and continuous variables were analyzed using the independent t-test or Mann-Whitney U-rank test. Survival curves and disease-free intervals were obtained using the Kaplan-Meier method. The differences in OS and DFS rates were assessed using the log-rank test. P-values less than 0.05 were considered to indicate statistical significance.
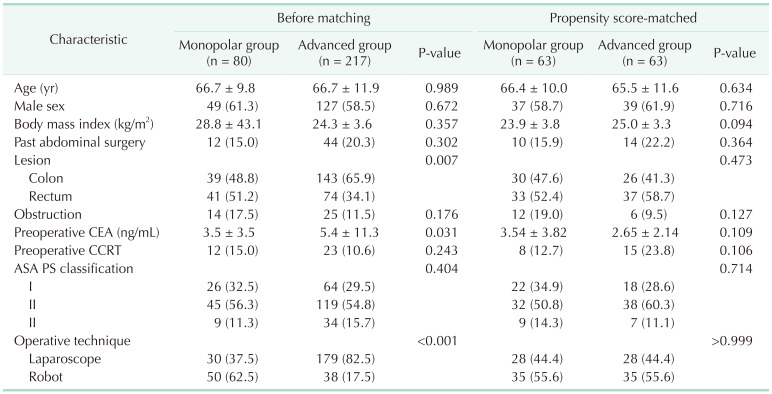
We estimated propensity scores with logistic regression to mitigate the confounding influence of the following covariates: tumor lesion, preoperative level of CEA, and operation technique, because these variables were significantly different between the monopolar energy group and advanced energy group in patient baseline characteristics. P-value for the Hosmer-Lemeshow goodness-of-fit test of propensity score matching model was 0.382. After matching, no significant differences in the baseline characteristics were shown.
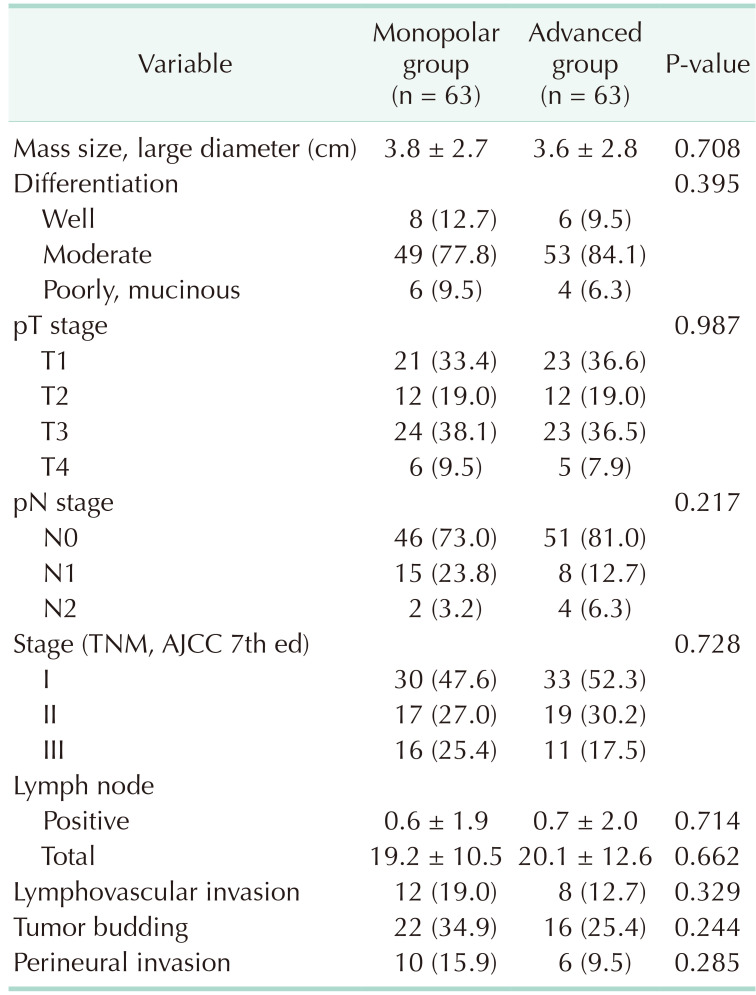
Demographic characteristics were similar between the 2 study groups such as age, sex, body mass index, tumor’s location, preoperative existence of obstruction, preoperative CEA, American Society of Anesthesiologists (ASA) physical status (PS) classification, and operation technique after the propensity score matching was performed (Table 1).
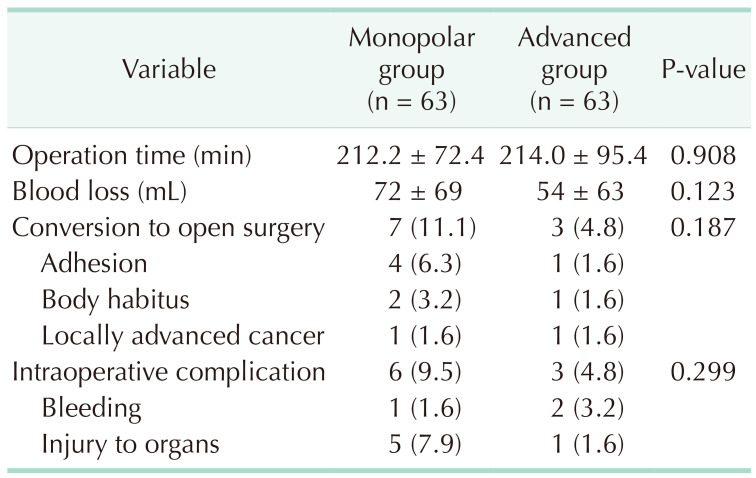
The median operation time was equivalent between the monopolar energy group and the advanced energy group (212.2 minutes vs. 214.0 minutes, P = 0.908). The amount of blood loss (72 mL vs. 54 mL, P = 0.123), conversion cases to open surgery (11.1% vs. 4.8%, P = 0.187), and intraoperative complications (9.5% vs. 4.8%, P = 0.299) were higher in the monopolar energy group, but the differences were not statistically significant. In the monopolar energy group, the majority cause of conversion was severe adhesion (4 cases, 6.3%), and most intraoperative complications were injury to adjacent organs (5 cases, 7.9%) (Table 2).
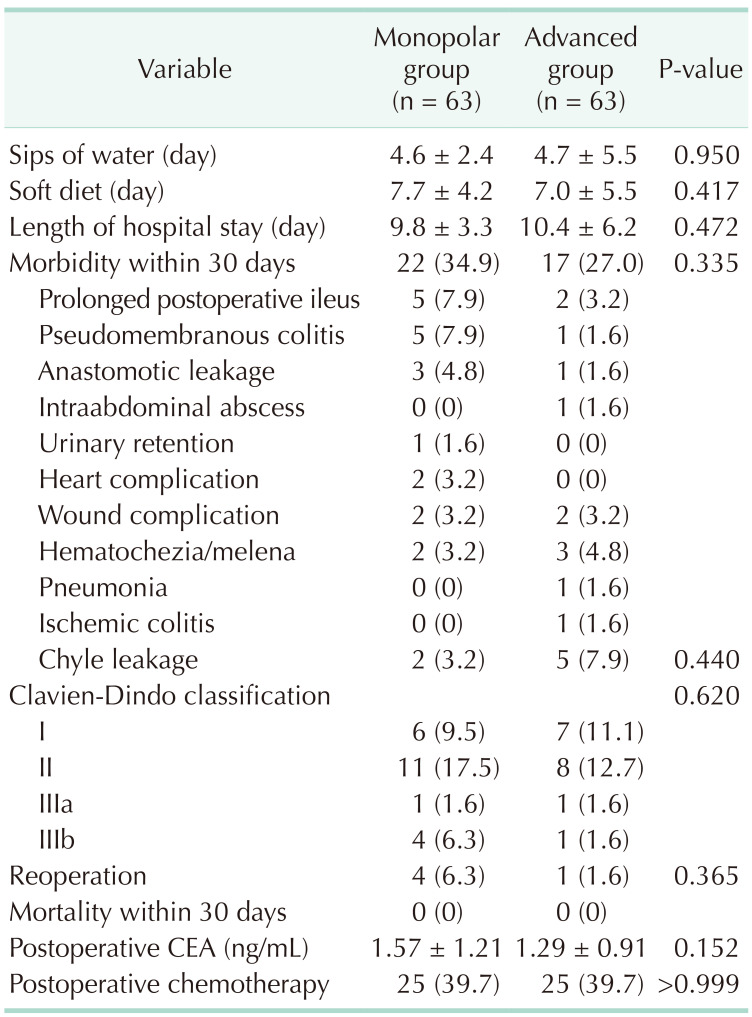
There were no apparent differences in the tolerance of diet and length of hospital stay between the 2 groups. The morbidity rates within 30 days (34.9% in the monopolar energy group, 27.0% in the advanced energy group; P = 0.335) and their severity according to the Clavien-Dindo classification (P = 0.620) were comparable between the groups. Anastomotic site leakage occurred in 4 patients (3 patients in the monopolar energy group and 1 patient in the advanced energy group) who received reoperation as primary repair or diverting stoma. On the other hand, anastomotic site stenosis occurred in 1 patient of the monopolar energy group; who received surgical dilatation of stricture. No mortalities occurred within 30 days in both study groups. The same proportion of patients from each group (39.7%) received postoperative adjuvant chemotherapy (Table 3).
There were no significant differences in the pathologic outcome of resected tumors, such as mass size, tumor differentiation, pathologic T or N stage, lymphovascular invasion, tumor budding, and perineural invasion. The median numbers of harvested lymph nodes (monopolar, 19.2 vs. energy device, 20.1; P = 0.662) and the number of tumor-positive lymph nodes (monopolar, 0.6 vs. energy device, 0.7; P = 0.714) were similar in the 2 groups (Table 4).
In our study, median lengths of follow-up periods were 52.0 months for the whole study population; 52.9 months in the monopolar energy group and 51.1 months in the advanced energy group. Overall, 16 patients had recurrence (11 in the monopolar energy group, 5 in the advanced energy group), and 14 patients died (9 in the monopolar energy group, 5 in the advanced energy group). During the follow-up periods, the 5-year OS rates of the monopolar and advanced energy groups were 84.6% and 91.6% (P = 0.276), and the 5-year DFS rates were 78.0% and 84.6% (P = 0.328), respectively. Recurrence rate (17.5% vs. 7.9%) was higher in the monopolar energy group than the advanced energy group, and more systemic recurrences occurred in the monopolar energy group (8 cases, 12.7% vs. 4 cases, 6.3%), though these distributions of recurrence were not significantly different between the 2 groups (P = 0.108 and P = 0.332, respectively).
We hypothesized that advanced energy devices may affect oncologic outcomes as they have greater tumoricidal and sealing effects around the lymphovascular chain than conventional monopolar devices. To investigate this, we performed a subgroup analysis of stage III patients (17 patients in the monopolar energy group and 12 patients in the advanced energy group). During the same follow-up period, the 5-year OS rates (76.5% and 83.3%, respectively; P = 0.682) and 5-year DFS rates (70.1% and 75.0%, respectively; P = 0.736) were comparable between the 2 subgroups (Table 5).
The current study showed that postoperative clinical outcomes, including operation time, amount of blood loss, length of hospital stay, and complication rates, did not differ between the groups using conventional monopolar devices and advanced surgical energy devices. In addition, we found that the advanced energy device did not affect long-term oncologic outcomes, such as 5-year OS and DFS rates. As far as we know, this study is the first study on the long-term oncologic outcome of advanced energy device in surgery of CRC. Furthermore, among previous studies, our study may have several strengths of propensity score matching to minimize selection bias.
The advanced surgical energy devices were designed to be multifunctioning: as grasper, dissector, cutter, or coagulator in a single device [78]. Some studies from randomized clinical trials show intraoperative advantages, including lower intraoperative blood loss and shorter operative time in patients undergoing MIS of CRC with ultrasonic shears or advanced bipolar vessel sealers than conventional monopolar devices [811]. In an ex vivo study using human pulmonary artery branches, vascular sealing by advanced energy devices was effective and able to sustain high intraluminal bursting pressures [15].
Therefore, surgeons may think that an advanced energy device can be superior in operation time and amount of blood loss because it produces higher burst pressure of dissected arteries as well as a significantly faster tissue dissection time [71617]. However, in our study, there was no significant difference in operative outcomes between the 2 study groups. A previous retrospective study, which included patients who underwent colorectal resection, reported similar results to ours in that no significant differences were observed in operative time, conversion to open surgery rates, the incidence of device-related injury to intraabdominal organs, and postoperative morbidity between conventional monopolar device group and advanced energy device group [13].
The number of dissected lymph nodes and the ratio of involved vs. dissected lymph nodes have been used as markers for the quality of surgery and histopathological evaluation [1819]. Recent studies suggested that advanced energy devices may have superiority in radical lymphadenectomy because of their higher bursting pressure and versatility in hemostasis, sealing/coagulation, cutting, dissection, and tissue manipulation in various ex vivo models using porcine vessels [720]. In the current study, the number of total harvested lymph nodes and cancer-positive lymph nodes were not different between the 2 study groups. We believe that the oncologic principle of radical lymphadenectomy with training in dedicated surgical skills is more important for the quality of cancer surgery than the surgical instrument itself, although innovative instruments have been developed.
The optimal technique for curative resection of colon cancer includes high ligation of the mesenteric vessels, wide excision of the colonic mesentery, and prevention of tumor cell spillage [21]. Especially, during lymph node dissection in CRC, surgeons should avoid spillage of residual tumor cells from lymphatic leakage because it can cause tumor recurrence and produce a poor prognosis [2223]. It can be said that an advanced surgical energy device is more advantageous in the treatment of microscopic tumor cells around lymphovascular structures. We hypothesized that advanced surgical energy devices could allow less microscopic cancer cell spillage and have a more tumoricidal effect during lymph node dissection, and impact on oncological outcomes. However, there was no significant difference in postoperative chyle leakage rate and long-term oncologic outcomes between the monopolar energy group and advanced energy group, even if in the subgroup analysis of stage III patients. Additional preclinical studies and clinical studies using larger sample sizes on this issue are needed in the future.
The current study has several limitations, including its retrospective nature, small sample size, lack of multicenter data, and selection bias resulting from surgeons’ individual preference-based selection of surgical energy devices. Thus, further large long-term randomized prospective studies and objective selection criteria for each device should be set up to demonstrate any significant benefit of using advanced surgical energy devices in MIS for CRC.
In conclusion, the use of advanced surgical energy devices did not show a significant impact on operative and long-term outcomes compared with the conventional monopolar device in MIS for CRC.
Notes
References
1. Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy). Surg Laparosc Endosc. 1991; 1:144–150. PMID: 1688289.
2. Bae SU, Jeong WK, Baek SK. Robot-assisted colectomy for left-sided colon cancer: comparison of reduced-port and conventional multi-port robotic surgery. J Laparoendosc Adv Surg Tech A. 2017; 27:398–403. PMID: 27870592.

3. Jaloun HE, Lee IK, Kim MK, Sung NY, Turkistani SA, Park SM, et al. Influence of the enhanced recovery after surgery protocol on postoperative inflammation and short-term postoperative surgical outcomes after colorectal cancer surgery. Ann Coloproctol. 2020; 36:264–272. PMID: 32674557.

4. Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009; 10:44–52. PMID: 19071061.

5. Juo YY, Hyder O, Haider AH, Camp M, Lidor A, Ahuja N. Is minimally invasive colon resection better than traditional approaches?: first comprehensive national examination with propensity score matching. JAMA Surg. 2014; 149:177–184. PMID: 24352653.

6. Oh CK, Huh JW, Lee YJ, Choi MS, Pyo DH, Lee SC, et al. Long-term oncologic outcome of postoperative complications after colorectal cancer surgery. Ann Coloproctol. 2020; 36:273–280. PMID: 32054256.

7. Milsom J, Trencheva K, Monette S, Pavoor R, Shukla P, Ma J, et al. Evaluation of the safety, efficacy, and versatility of a new surgical energy device (THUNDERBEAT) in comparison with Harmonic ACE, LigaSure V, and EnSeal devices in a porcine model. J Laparoendosc Adv Surg Tech A. 2012; 22:378–386. PMID: 22364404.

8. Allaix ME, Furnée EJ, Arezzo A, Mistrangelo M, Morino M. Energy sources for laparoscopic colorectal surgery: is one better than the others? J Laparoendosc Adv Surg Tech A. 2016; 26:264–269. PMID: 26986017.

9. Hotta T, Takifuji K, Yokoyama S, Matsuda K, Higashiguchi T, Tominaga T, et al. Literature review of the energy sources for performing laparoscopic colorectal surgery. World J Gastrointest Surg. 2012; 4:1–8. PMID: 22347536.

10. Suhardja TS, Norhadi S, Ee E, Hodgkins B. Comparison of the Thunderbeat and other energy devices in laparoscopic colorectal resection: a single-center experience. J Laparoendosc Adv Surg Tech A. 2018; 28:1417–1421. PMID: 29870293.

11. Tou S, Malik AI, Wexner SD, Nelson RL. Energy source instruments for laparoscopic colectomy. Cochrane Database Syst Rev. 2011; (5):CD007886. PMID: 21563161.

12. Milsom JW, Trencheva K, Sonoda T, Nandakumar G, Shukla PJ, Lee S. A prospective trial evaluating the clinical performance of a novel surgical energy device in laparoscopic colon surgery. Surg Endosc. 2015; 29:1161–1166. PMID: 25159634.

13. Allaix ME, Arezzo A, Giraudo G, Arolfo S, Mistrangelo M, Morino M. The Thunderbeat and other energy devices in laparoscopic colorectal resections: analysis of outcomes and costs. J Laparoendosc Adv Surg Tech A. 2017; 27:1225–1229. PMID: 27420752.

14. Hohenberger W, Weber K, Matzel K, Papadopoulos T, Merkel S. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation: technical notes and outcome. Colorectal Dis. 2009; 11:354–365. PMID: 19016817.
15. Liberman M, Khereba M, Goudie E, Kazakov J, Thiffault V, Lafontaine E, et al. Pilot study of pulmonary arterial branch sealing using energy devices in an ex vivo model. J Thorac Cardiovasc Surg. 2014; 148:3219–3223. PMID: 25125207.

16. Seehofer D, Mogl M, Boas-Knoop S, Unger J, Schirmeier A, Chopra S, et al. Safety and efficacy of new integrated bipolar and ultrasonic scissors compared to conventional laparoscopic 5-mm sealing and cutting instruments. Surg Endosc. 2012; 26:2541–2549. PMID: 22447285.

17. Fagotti A, Vizzielli G, Fanfani F, Gallotta V, Rossitto C, Costantini B, et al. Randomized study comparing use of THUNDERBEAT technology vs standard electrosurgery during laparoscopic radical hysterectomy and pelvic lymphadenectomy for gynecologic cancer. J Minim Invasive Gynecol. 2014; 21:447–453. PMID: 24325899.

18. Akagi Y, Adachi Y, Kinugasa T, Oka Y, Mizobe T, Shirouzu K. Lymph node evaluation and survival in colorectal cancer: review of population-based, prospective studies. Anticancer Res. 2013; 33:2839–2847. PMID: 23780968.
19. Nakamura Y, Hokuto D, Koyama F, Matsuo Y, Nomi T, Yoshikawa T, et al. The prognosis and recurrence pattern of right- and left-sided colon cancer in stage II, stage III, and liver metastasis after curative resection. Ann Coloproctol. 2021; 37:326–336. PMID: 32972100.

20. Tanaka R, Gitelis M, Meiselman D, Abar B, Zapf M, Carbray J, et al. Evaluation of vessel sealing performance among ultrasonic devices in a porcine model. Surg Innov. 2015; 22:338–343. PMID: 25851145.

21. Liang J, Fazio V, Lavery I, Remzi F, Hull T, Strong S, et al. Primacy of surgery for colorectal cancer. Br J Surg. 2015; 102:847–852. PMID: 25832316.

22. Enríquez-Navascués JM, Borda N, Lizerazu A, Placer C, Elosegui JL, Ciria JP, et al. Patterns of local recurrence in rectal cancer after a multidisciplinary approach. World J Gastroenterol. 2011; 17:1674–1684. PMID: 21483626.

23. Kessler H, Hohenberger W. Extended lymphadenectomy in colon cancer is crucial. World J Surg. 2013; 37:1789–1798. PMID: 23754141.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download