Abstract
Purpose
Hungry bone syndrome after parathyroidectomy is an important clinical problem in patients on maintenance hemodialysis. We examined the effect of an enhanced recovery after surgery (ERAS) program on the incidence of hungry bone syndrome after parathyroidectomy in this population.
Methods
This single-institution, retrospective study analyzed 108 patients on hemodialysis who underwent parathyroidectomy for secondary hyperparathyroidism. Patients were classified into the pre-ERAS (n = 52) and post-ERAS (n = 56) groups. The ERAS program identified high-risk patients and enforced aggressive measures to normalize calcium levels following parathyroidectomy.
Results
There was no significant difference in age, sex, body weight, presenting symptoms, preoperative calcium and alkaline phosphatase levels, postoperative intact parathyroid levels, postoperative calcium levels at 1 and 24 hours after parathyroidectomy, and 30-day readmission rates between the groups. The post-ERAS group had significantly higher levels of postoperative calcium at 48 and 72 hours after parathyroidectomy, but a lower incidence of hungry bone syndrome and shorter postoperative length of stay. Patients with hungry bone syndrome had higher preoperative levels of alkaline phosphatase and intact parathyroid, longer postoperative length of stay, and were less likely to have been part of the ERAS program. High preoperative alkaline phosphatase levels and absence of the ERAS program were independent risk factors for hungry bone syndrome after parathyroidectomy.
Secondary hyperparathyroidism (SHPT) is a common complication of end-stage renal disease (ESRD) and affects most patients undergoing hemodialysis maintenance. Elevated parathyroid hormone (PTH) levels are associated with an increased risk for osteodystrophy resulting in fractures, anemia hyporesponsive to erythropoietin therapy, vascular and tissue calcification, and mortality [1]. The Kidney Disease Outcomes Quality Initiative (KDOQI) clinical practice guidelines recommend pharmacologic and surgical therapies, such as parathyroidectomy (PTX) for SHPT [2], for mineral bone disorders; however, the benefit of pharmacologic therapy as the first-line therapy for SHPT remains unclear because the incidence of PTX among patients with SHPT has remained stable despite advancements in pharmacologic therapy [3]. While PTX improves survival rates and reduces all-cause mortality by 15%–57% [1], it is associated with an increased short-term risk for mortality; the 30-day mortality of PTX ranges from 0.8% to 3.1% [4]. Patients who undergo PTX for SHPT also have a higher risk for readmission than patients who undergo PTX for primary hyperparathyroidism [5]. The increased risk for short-term mortality and readmission may be attributed to poorer baseline functions among patients with SHPT because they are more prone to endocrine and electrolyte abnormalities. The most common complications of PTX include hypocalcemia and hungry bone syndrome (HBS) [146]. PTX reduces serum PTH levels, which promotes osteoblastic calcium uptake and HBS [7]. Patients with HBS have a significantly longer length of stay (LOS) [89] and higher 30-day readmissions [59] than patients without HBS. While HBS can be managed with calcium and active vitamin D supplementation, it is associated with life-threatening complications, such as mental status changes, tetany, laryngeal stridor, and cardiac arrhythmias [78].
The enhanced recovery after surgery (ERAS) program was initially utilized in colorectal surgery but has since been adopted across many surgical disciplines. The ERAS program aims to improve recovery, shorten LOS, and reduce complications, readmissions, and costs [1011]. Studies on the benefits of ERAS programs in patients undergoing PTX are limited; existing studies only demonstrated that ERAS programs reduced opioid use following PTX [1213]. Therefore, the present study examined whether a customized ERAS program reduced the incidence of HBS among patients on maintenance dialysis who underwent PTX for SHPT.
The study was approved by the Ethics Committee of Xiangyang Central Hospital (No. XYZXYY#2020–11) and conducted in accordance with the Declaration of Helsinki. All patients provided written informed consent.
This single-institution retrospective study evaluated 108 patients on hemodialysis who underwent total PTX without autotransplantation for SHPT in Xiangyang Central Hospital between May 2020 and June 2021. Patients who (1) were >18 years old; (2) were on maintenance hemodialysis for at least 1 year; (3) met the diagnostic criteria for refractory SHPT, which included persistently elevated intact parathyroid hormone (iPTH) of >800 pg/mL for at least 6 months despite pharmacologic therapy (vitamin D analogs, cinacalcet); and (4) demonstrated nodular parathyroid hyperplasia on ultrasound, were included in this study. Patients with a history of PTX or who were lost to follow-up were excluded. The customized ERAS program was implemented at our hospital in January 2021. Patients treated prior to January 2021 comprised the pre-ERAS group, whereas patients treated from January 2021 comprised the post-ERAS group.
Laboratory parameters, such as the levels of albumin-corrected serum calcium, ALP, and iPTH, were evaluated upon admission. Serum calcium levels were monitored at 1, 24, 48, and 72 hours intervals after PTX, whereas serum ALP and iPTH levels were reevaluated 24 hours after PTX. Patient characteristics, including age, sex, and body weight, and clinical data, such as postoperative LOS, complications, and 30-day readmission rates, were collected. Postoperative LOS was defined as the number of days between surgery and discharge. HBS was defined as a serum calcium level of <2.1 mmol/L at 72 hours postoperatively [8].
We customized the ERAS program for HBS. The safety and efficacy of each item were evaluated through literature review. The final protocol was approved by the surgical, hemodialysis, and nursing teams of Xiangyang Central Hospital. The revised ERAS program is shown in Table 1. Preoperatively, patients who were at high risk for HBS, including those who were <45 years old and had preoperative serum ALP levels greater than 3 times the upper limit of normal (>375 U/L) or a serum PTH of >2,000 pg/mL [171415] were identified. These patients were educated on the purpose, flow, and benefits of the ERAS program, and their serum calcium levels were normalized close to the upper limit of normal (2.5 mmol/L) with calcium carbonate (1 g, thrice daily) and calcitriol (1 µg, once daily). Intraoperatively, the patients were placed on sodium chloride and water overload restriction. A central venous catheter for postoperative intravenous calcium supplementation was also placed. Immediate intravenous calcium supplementation was initiated in the postoperative period regardless of pre- and postoperative calcium levels. Ten percent calcium gluconate was diluted with equal parts of 5% glucose to prevent volume overload. Calcium carbonate (2 g, thrice daily) and calcitriol (2 µg, once daily) were administered until normal ionized calcium levels were reached. High-calcium dialysate (1.75 mmol/L calcium) was also administered when needed, along with early oral calcium-rich feeding. We followed the KDOQI clinical practice guidelines for bone metabolism and diseases, and measured the blood level of ionized calcium every 6 hours for the first 48 to 72 hours after surgery, and then twice daily until they had stabilized [2].
Statistical analysis was performed with the SigmaPlot 13 software (Systat Software Inc., San Jose, CA, USA). The Shapiro-Wilk test was used to assess the normality of continuous variables. Data are described as number (percentage), median (interquartile range), or mean ± standard deviation. Comparison between groups was performed using the 1-way analysis of variance or Student t-test, and categorical variables were analyzed using the chi-square test. A P-value of <0.05 was considered reflective of statistical significance.
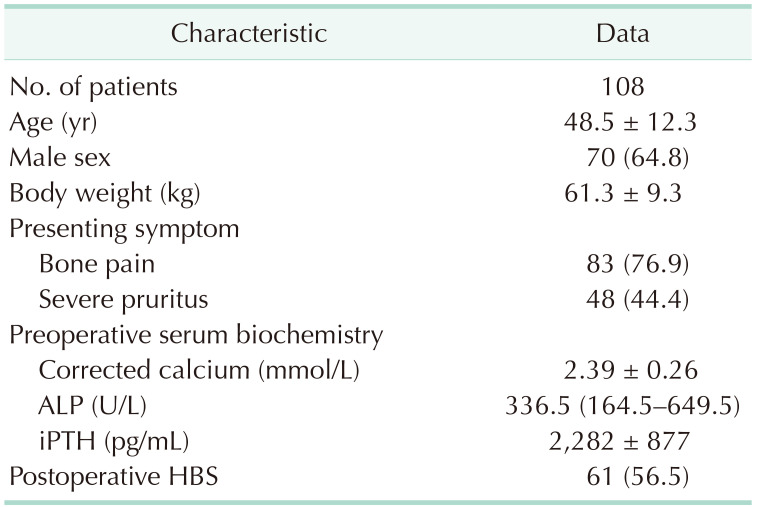
The patient and clinical characteristics are listed in Table 2. We analyzed 108 patients (70, male and 38, female) with a mean age of 48.5 ± 12.3 years. The mean body weight was 61.3 ± 9.3 kg. The most common presenting symptoms included bone pain and severe pruritus, which were documented in 76.9% and 44.4% of patients, respectively. The median of preoperative serum ALP and mean of iPTH levels were 336.5 U/L (164.5–649.5 U/L) and 2,282 ± 877 pg/mL, respectively. Postoperative HBS was diagnosed in 61 patients (56.5%).
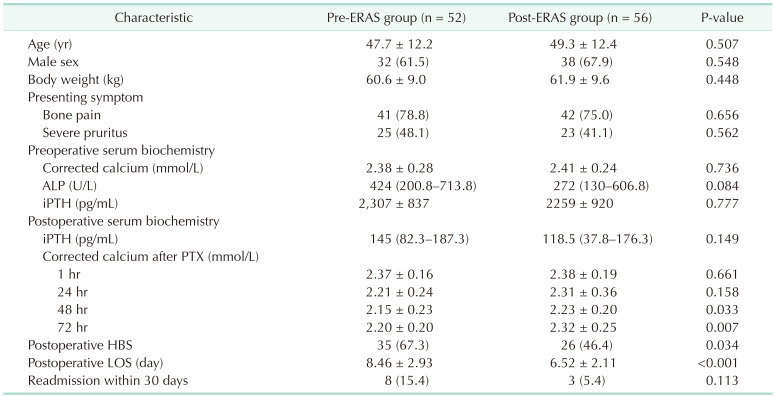
Fifty-two (48.1%) and 56 patients (51.9%) comprised the pre-ERAS and post-ERAS groups, respectively. The clinical features of each group are shown in Table 3. There were no significant differences in age, sex, body weight, presenting symptoms, preoperative serum calcium and ALP levels, pre- and postoperative iPTH levels, and 30-day readmission rates between the groups. While there was no significant difference in the postoperative serum calcium levels at 1 and 24 hours, serum calcium levels at 48 and 72 hours differed significantly between the groups. The post-ERAS group demonstrated a lower incidence of HBS and shorter postoperative LOS than the pre-ERAS group.
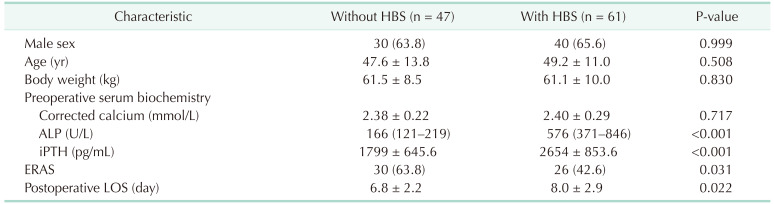
Patients with HBS had higher preoperative ALP and iPTH levels and a longer postoperative LOS than patients without HBS. There were significantly fewer patients with than without HBS in the post-ERAS group (42.6% vs. 63.8%, P = 0.030) (Table 4).
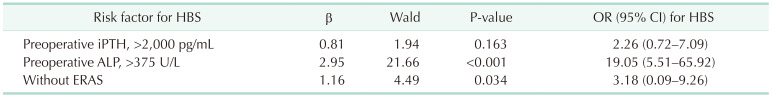
Univariate analysis demonstrated that preoperative serum ALP and iPTH levels and the ERAS program influenced the risk for HBS. Multivariate logistic regression analysis demonstrated that a preoperative serum ALP level of >375 U/L and absence of the ERAS program were independent risk factors for HBS (Table 5).
Studies have shown that the ERAS program improves outcomes across a wide range of surgical subspecialties; however, its role in patients on maintenance hemodialysis who underwent PTX remains unclear. Our study indicated the clear benefits of a customized ERAS program in this group of patients, as it lowered the incidence of HBS and shortened the postoperative LOS. High preoperative serum ALP levels and the absence of the ERAS program were independent risk factors for HBS.
SHPT is a common complication among patients undergoing hemodialysis. SHPT is treated with vitamin D analogs and calcimimetic medication, but the benefits of this approach are controversial. A previous study demonstrated that vitamin D analogs do not improve survivability among patients with ESRD [16]. The EVOLVE (Evaluation of Cinacalcet Hydrochloride Therapy to Lower CardioVascular Events) study indicated that cinacalcet did not significantly reduce all-cause mortality or major cardiovascular events in patients with SHPT compared with placebo [17]. According to the KDOQI clinical practice guidelines, PTX should be considered in patients with refractory SHPT [12]. PTX effectively reduces serum PTH levels, which resolves disease-related symptoms such as severe pruritis and bone pain, decreases fracture risk, and improves left ventricular function and overall quality of life [41819]. PTX is an effective treatment for SHPT but is associated with postoperative hypocalcemia and HBS. PTX alters the balance between bone formation and resorption, because PTX results in an abrupt decrease in serum PTH levels, which inhibits osteoclast absorption. However, osteoblastic activity continues, which results in an influx of calcium into bone, prompting hypocalcemia and HBS [7]. HBS is characterized by rapid, severe, and long-term hypocalcemia. Previous studies documented HBS in 28%–88% of patients who underwent PTX for SHPT [8]. Our data identified HBS in 56.5% of patients who underwent PTX for SHPT, which is consistent with values revealed by previous research [8].
HBS is associated with a longer LOS, more unplanned readmissions within 30 days, and higher mortality. A study that analyzed 2,756 patients with chronic kidney disease (>50% had ESRD) who underwent PTX reported an unplanned 30-day readmission rate of 17.2%; hypocalcemia and HBS were the primary cause of 40% of the readmissions [5]. A more recent analysis of 1,846 patients on hemodialysis who underwent PTX for SHPT reported a 30-day readmission rate of 15%; hypocalcemia and HBS accounted for 47% of the readmissions in this study [9]. Our study demonstrated that patients with HBS had a longer postoperative LOS, which indicated that the customized ERAS program significantly reduced the incidence of HBS and shortened the postoperative LOS. While the difference was not statistically significant, the 30-day readmission rate was lower in the post-ERAS group than in the pre-ERAS group (5.4% vs. 15.4%). The absence of significance may be due to the relatively small sample sizes in each group.
Previous studies proposed that HBS was associated with high preoperative serum ALP and iPTH levels [81420], which is in accordance with our findings. We also demonstrated that high preoperative serum ALP levels were an independent risk factor for HBS. While patients with HBS had higher preoperative serum iPTH levels, higher preoperative serum iPTH levels were not an independent risk factor for HBS.
There is limited data on the role of ERAS in our study population. Two publications examined the role of ERAS in decreasing opioid use following PTX [1213], but these studies did not distinguish primary hyperparathyroidism from SHPT. To the best of our knowledge, this is the first study to evaluate the role of an ERAS program in reducing the incidence of HBS among patients on hemodialysis who underwent PTX [1213]. Patients on hemodialysis for ESRD have an increased risk for electrolyte imbalances, and previous studies demonstrated an association between renal impairment and hemodialysis and readmission after PTX [2122]. The ERAS program must be customized for patients on hemodialysis because they have unique risk factors. Proper treatment of SHPT in developing countries such as China is complicated due to economic factors and insufficient knowledge on SHPT. Most patients, like the patients in this study, present with severe symptoms, including bone pain, severe pruritus, and calciphylaxis. The preoperative serum iPTH levels in these patients were also markedly above the threshold (>800 pg/mL) specified by the KDOQI clinical practice guidelines [2324].
This study has several limitations. First, it followed a retrospective, nonrandomized design. Second, we examined a small sample size of patients from a single center. Third, all patients came from China, and presented with severe symptoms and delayed surgical opportunity. The results of this study may therefore not be applicable outside of mainland China.
Our study demonstrated that a customized ERAS program effectively reduced the incidence of HBS and shortened the postoperative LOS among patients on hemodialysis who underwent PTX for SHPT. Absence of the ERAS program was an independent risk factor for HBS. We intend to monitor the results of our customized ERAS program and propose future improvements.
References
1. Lau WL, Obi Y, Kalantar-Zadeh K. Parathyroidectomy in the management of secondary hyperparathyroidism. Clin J Am Soc Nephrol. 2018; 13:952–961. PMID: 29523679.
2. Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Update Work Group. KDIGO 2017 clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl (2011). 2017; 7:1–59. PMID: 30675420.
3. Kim SM, Long J, Montez-Rath ME, Leonard MB, Norton JA, Chertow GM. Rates and outcomes of parathyroidectomy for secondary hyperparathyroidism in the United States. Clin J Am Soc Nephrol. 2016; 11:1260–1267. PMID: 27269300.

4. Steinl GK, Kuo JH. Surgical management of secondary hyperparathyroidism. Kidney Int Rep. 2020; 6:254–264. PMID: 33615051.

5. Ferrandino R, Roof S, Ma Y, Chan L, Poojary P, Saha A, et al. Unplanned 30-day readmissions after parathyroidectomy in patients with chronic kidney disease: a nationwide analysis. Otolaryngol Head Neck Surg. 2017; 157:955–965. PMID: 28949797.

6. Tan PG, Ab Hadi IS, Zahari Z, Yahya MM, Wan Zain WZ, Wong MP, et al. Predictors of early postoperative hypocalcemia after total parathyroidectomy in renal hyperparathyroidism. Ann Surg Treat Res. 2020; 98:1–6. PMID: 31909044.

7. Jain N, Reilly RF. Hungry bone syndrome. Curr Opin Nephrol Hypertens. 2017; 26:250–255. PMID: 28375869.

8. Kritmetapak K, Kongpetch S, Chotmongkol W, Raruenrom Y, Sangkhamanon S, Pongchaiyakul C. Incidence of and risk factors for post-parathyroidectomy hungry bone syndrome in patients with secondary hyperparathyroidism. Ren Fail. 2020; 42:1118–1126. PMID: 33143476.

9. Stefanova D, Ullmann TM, Limberg J, Moore M, Beninato T, Zarnegar R, et al. Risk factors for prolonged length of stay and readmission after parathyroidectomy for renal secondary hyperparathyroidism. World J Surg. 2020; 44:3751–3760. PMID: 32737558.

10. Ljungqvist O, Scott M, Fearon KC. Enhanced recovery after surgery: a review. JAMA Surg. 2017; 152:292–298. PMID: 28097305.
11. Wang C, Lai Y, Li P, Su J, Che G. Influence of enhanced recovery after surgery (ERAS) on patients receiving lung resection: a retrospective study of 1749 cases. BMC Surg. 2021; 21:115. PMID: 33676488.

12. Yip L, Carty SE, Holder-Murray JM, Recker A, Nicholson KJ, Boisen ML, et al. A specific enhanced recovery protocol decreases opioid use after thyroid and parathyroid surgery. Surgery. 2021; 169:197–201. PMID: 32690334.

13. Lide RC, Creighton EW, Yeh J, Troughton M, Hollowoa B, Merrill T, et al. Opioid reduction in ambulatory thyroid and parathyroid surgery after implementing enhanced recovery after surgery protocol. Head Neck. 2021; 43:1545–1552. PMID: 33502069.

14. Ho LY, Wong PN, Sin HK, Wong YY, Lo KC, Chan SF, et al. Risk factors and clinical course of hungry bone syndrome after total parathyroidectomy in dialysis patients with secondary hyperparathyroidism. BMC Nephrol. 2017; 18:12. PMID: 28073343.

15. Sun X, Zhang X, Lu Y, Zhang L, Yang M. Risk factors for severe hypocalcemia after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Sci Rep. 2018; 8:7743. PMID: 29773914.

16. Cantor TL. Lack of evidence for administering vitamin D analogs to kidney failure patients to improve survivability. Clin Nephrol. 2009; 72:97–104. PMID: 19640366.

17. EVOLVE Trial Investigators. Chertow GM, Block GA, Correa-Rotter R, Drüeke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012; 367:2482–2494. PMID: 23121374.

18. Zhang Y, Lu Y, Feng S, Zhan Z, Shen H. Evaluation of laboratory parameters and symptoms after parathyroidectomy in dialysis patients with secondary hyperparathyroidism. Ren Fail. 2019; 41:921–929. PMID: 31573378.

19. Cheng SP, Lee JJ, Liu TP, Yang TL, Chen HH, Wu CJ, et al. Parathyroidectomy improves symptomatology and quality of life in patients with secondary hyperparathyroidism. Surgery. 2014; 155:320–328. PMID: 24035616.

20. Goldfarb M, Gondek SS, Lim SM, Farra JC, Nose V, Lew JI. Postoperative hungry bone syndrome in patients with secondary hyperparathyroidism of renal origin. World J Surg. 2012; 36:1314–1319. PMID: 22399154.

21. Mullen MG, LaPar DJ, Daniel SK, Turrentine FE, Hanks JB, Smith PW. Risk factors for 30-day hospital readmission after thyroidectomy and parathyroidectomy in the United States: an analysis of National Surgical Quality Improvement Program outcomes. Surgery. 2014; 156:1423–1431. PMID: 25456925.

22. Iannuzzi JC, Fleming FJ, Kelly KN, Ruan DT, Monson JR, Moalem J. Risk scoring can predict readmission after endocrine surgery. Surgery. 2014; 156:1432–1440. PMID: 25456927.

23. van der Plas W, Kruijff S, Sidhu SB, Delbridge LW, Sywak MS, Engelsman AF. Parathyroidectomy for patients with secondary hyperparathyroidism in a changing landscape for the management of end-stage renal disease. Surgery. 2021; 169:275–281. PMID: 33059930.

24. Jimeno-Fraile J, Cao H, Sancho-Insenser J, Lorente-Poch L, Sitges-Serra A. Muscle strength, physical performance, and metabolic changes after subtotal parathyroidectomy for secondary hyperparathyroidism. Surgery. 2021; 169:846–851. PMID: 33218703.

Table 1
Customized enhanced recovery after surgery protocol for hungry bone syndrome among patients on hemodialysis





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download