INTRODUCTION
Bleeding is one of the most common and potentially detrimental complications of surgery—leading to death in severe cases. In particular, liver surgery poses a higher bleeding risk than other surgeries. This is because patients undergoing liver surgery often possess reduced liver function compared to other patients. In addition, minor or major vessels must be dissected and ligated for liver resection. According to a published report, the average blood loss during liver surgery approached 700–1,200 mL [
1].
Therefore, to prevent bleeding during and after surgery, it is essential to investigate the bleeding risk before surgery. Various preoperative laboratory test parameters, including platelet levels, PT, aPTT, and bleeding time (BT) test, were used to evaluate the patient’s risk of bleeding before surgery. BT and platelet aggregation tests have been used to evaluate platelet function. However, the use of BT tests is gradually decreasing owing to its low sensitivity and high operator dependency [
2]. Platelet aggregation tests are relatively expensive and complex, although they are more sensitive than BT [
3]. Recently, platelet function tests using the platelet function analyzer (PFA-200; Siemens Healthineer, Munich, Germany) have been widely used as screening tools because of their ease of use and high sensitivity. In particular, it is possible to detect primary hemostasis abnormalities and monitor antiplatelet therapy [
45].
The usefulness of PFA as a preoperative screening tool has also been demonstrated and confirmed in heart surgery [
678]. However, its usefulness in patients undergoing liver resection has not yet been confirmed. In liver surgery, there is generally more bleeding than that in other surgeries, and many patients with reduced liver function are expected to exhibit reduced platelet levels or functions. For these reasons, PFA is expected to be useful. However, to date there are no reports confirming its utility. Therefore, our study focused on confirming the value of PFA in patients undergoing liver resection.
DISCUSSION
In heart patients with a high proportion of antiplatelet use, the usefulness of PFA in cardiac surgery has been demonstrated in several reports [
68]. However, the usefulness of routine PFA tests in other types of surgeries has not been proven. It has been reported that preoperative screening tests for PFA in all patients without a bleeding history delayed surgery and increased costs unnecessarily [
10]. Nevertheless, in clinical practice, PFA is often routinely used to evaluate the risk of bleeding during surgery, and this protocol is followed at our hospital. Using our hospital’s protocol, we attempted to confirm the usefulness of PFA before surgery in patients undergoing liver resection.
We attempted to confirm the usefulness of PFA in liver surgery in particular, because the hemostatic system is closely related to the clotting and fibrinolytic system mediated by liver parenchyma cells [
11]. Therefore, we tried to confirm 2 assumptions in this study. First, if liver resection is performed, liver parenchyma cells, which are responsible for clotting and the fibrinolytic system, are reduced. Therefore, we assumed that patients with abnormal platelet function on PFA would exhibit greater calculated blood loss during surgery. Second, we hypothesized that patients scheduled for liver resection for HCC would have impaired liver function compared to healthy individuals. Therefore, we attempted to find an association between preoperative liver function and PFA.
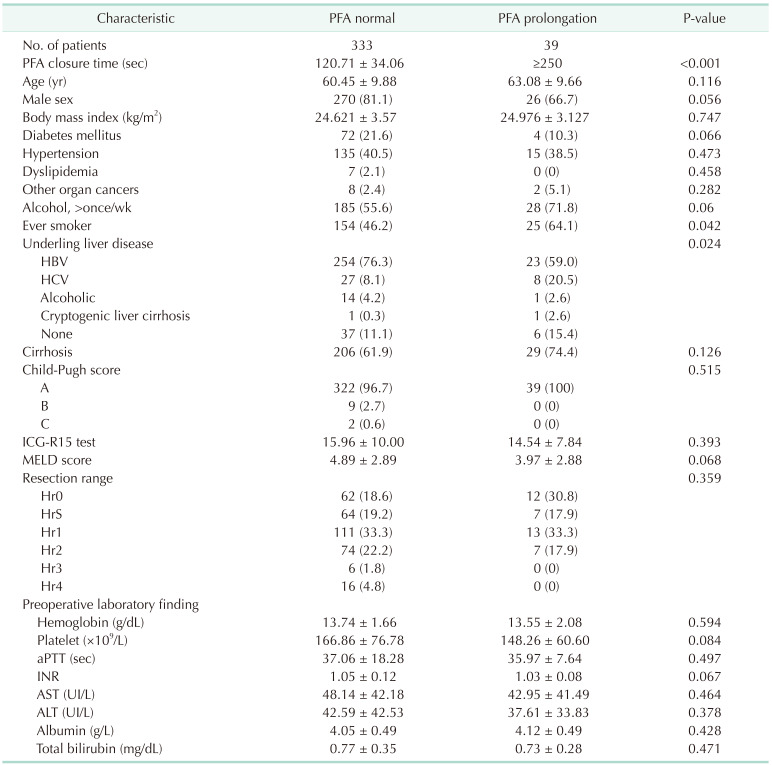
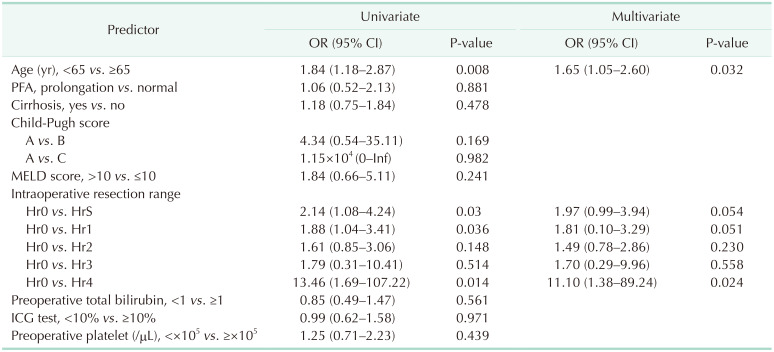
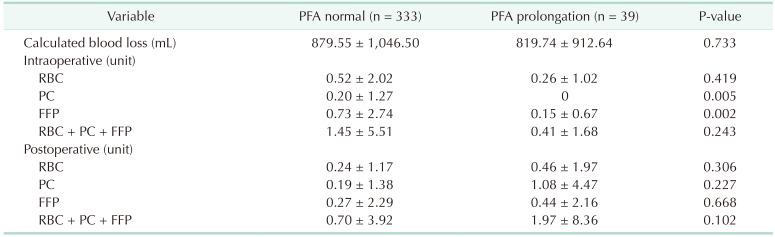
In our study, 10.5% of all patients exhibited prolonged PFA, and these patients were compared with the normal PFA group. However, there were no differences regarding calculated blood loss, intraoperative and postoperative RBC transfusions between the 2 groups. Further, multivariate analysis revealed that PFA did not impact a calculated blood loss. Resection range and age was the only predictive factor for calculated blood loss. Also regarding FFP, the normal PFA group showed a tendency to receive more transfusions than the prolonged PFA group. We supposed the reason is that the normal PFA group included more patients with extended sectionectomy than the PFA prolongation group. In liver cirrhosis patients, calculated blood loss and blood transfusion were similar to that of the total cohort. Also, hepatic resection was the only predictive risk factor for calculated blood loss. However, postoperative PC was more transfused in the PFA prolongation group. In liver cirrhosis patients, platelet function could be more dysfunctional and affect the postoperative needed PC transfusion. To prove it, further study was needed due to the small size of our study.
Other Korean studies on the usefulness of PFA before surgery reported similar results. Lee and Kim [
12] did not confirm the usefulness of PFA in endoscopic sinus surgery and concluded that the routine use of PFA is not recommended in endoscopic sinus surgery. Jeon et al. [
13] reported that PFA abnormalities in total knee arthroplasty were not related to postoperative bleeding. Yu et al. [
14] argued that PFA was useful for various surgeries in 703 patients; however, in this study, although patients with increased PFA closure time exhibited a higher rate of transfusion than those without increased PFA closure time, this was not confirmed by multivariate analysis. In this study, surgery was divided according to the surgical department, but we believe that even if it is an operation in the same area, the effect on a calculated blood loss may be different depending on the type and severity. Therefore, to evaluate PFA, its usefulness should be investigated for each operation. Therefore, we investigated only patients who underwent liver resection for HCC. A strength of our study is that the sample size is larger than that of other studies investigating PFA in one type of surgery.
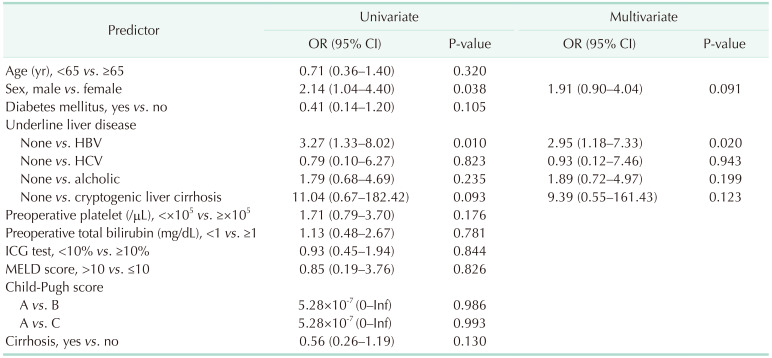
Our data did not confirm an effect of preoperative liver function on PFA values. The reason was that most of the patients had a satisfactory liver function, and most were classified as Child-Pugh A patients. In addition, each LFT value was within the normal range on average. Due to the nature of these cohorts, it was difficult to directly identify the factors that influence LFT on PFA. In multivariate analysis, hepatitis B was associated with PFA closure time. Hepatitis B was reported that it may associate with platelet production and dysfunction [
15]. However, in our data, the rate of hepatitis B is very high and the number of normal patients is small. Further study was needed for evaluating the association with hepatitis B and PFA closure time.
To confirm that LFT including ICG, INR, and bilirubin levels affect PFA, patients with a wider range of LFT values should be included. The effect of liver function on PFA may be better confirmed if patients who undergo transplantation due to hepatic failure are included. We did not include these patients in the cohort because one of our primary objectives was to determine whether PFA could predict intraoperative and postoperative bleeding. Because transplantation involves vascular surgery such as artery or portal vein anastomosis, the amount of bleeding is much higher than that of liver resection. Therefore, we believed that the effect of PFA on postoperative bleeding would be greater than that of other liver surgeries. Therefore, as a future study, the usefulness of PFA only for liver transplant patients with various liver functions should be investigated.
The limitations of our study are as follows. First, our cohort included a small number of hepatectomies involving more than 3 segments. Therefore, we were unable to compare the usefulness of PFA by distinguishing patients with a large number of liver resections compared to those with a small number of resections. Second, we could not confirm the change in PFA after surgery because we only determined the PFA preceding surgery. By determining the change in PFA after liver preservation in a future study, we would be able to better confirm the effect of liver resection on platelet function. Third, as mentioned above, it was difficult to confirm the effect of preoperative liver function on PFA because the rate of abnormal liver function was low.
In liver resection, we could not confirm the effect of PFA on a calculated blood loss or RBC transfusion. In addition, laboratory findings or assessments of tool-related liver function were not associated with prolonged PFA. In patients undergoing liver resection who are not managed on antiplatelet agents or do not have chronic kidney disease, the use of routine PFA is not recommended.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download