Abstract
Purpose
Breast volume is an important factor in breast reconstruction; however, the surgeon is expected to deliver the volume expectation with his aesthetic inspiration. Therefore, objective volumetry must be developed. This study aimed to conduct an MRI-based breast volumetric analysis. With periodic analysis of 2-stage breast reconstruction, we suggest the possibility of clinical use of breast volumetry in implant volume prediction.
Methods
This retrospective study included 140 patients who underwent unilateral 2-stage breast reconstruction (tissue expander followed by implant insertion) between January 1, 2017 and December 31, 2019. The MRI image was converted into a 3-dimensional image with a reconstruction program (A-VIEW, Coreline Soft). MRI image was obtained before the surgery and then at 1, 3, 6, 12, and 24 months postoperatively. The volume was automatically calculated.
Results
Compared with the preoperative volume, maximized volume and differences were noted at 1 month and minimized at 1 year. The correlation between MRI-based preoperative breast volumetry and the mastectomy specimen volume was 0.611. Volume difference between the MRI-based preoperative state and the implant volume showed a minimal difference at 1 year. The final implant size prediction formula was calculated using the 1-year postoperative volume (P < 0.001, R2 = 0.594).
Conclusion
To avoid breast reconstruction based solely on the surgeon’s subjective assessment, MRI-based breast volumetry could be a useful method to develop more scientific and objective breast reconstruction planning. We suggest a volume prediction formula that describes the relationship between the postoperative breast volume and the final breast implant size.
With the global increase in the number of breast cancer patients, breast cancer treatment has been focused on the original source; the breasts, their treatment, and reconstruction. Its importance and academic popularity have increased in plastic surgery. There are several reasons why the psychological impact of mastectomy is powerful among female cancer patients [1], as reported by Global Cancer Statistics 2020. The breast is not just a body part for women. It has an aesthetic and psychological significance; breast reconstruction has been proven to decrease the incidence of depression [2].
Recently, 2 methods for immediate prosthetic breast reconstruction have been proposed: 1-stage breast reconstruction or 2-stage breast reconstruction. The trend of increasing breast-conserving surgery among breast surgeons was followed by an increase in postmastectomy radiation treatment [3]. Two-stage breast reconstruction has been suggested as a popular method to obtain satisfiable breast aesthetics and radiation endurance on mastectomy skin flaps. Here, the reconstruction surgeon is required to produce the best volume expectation with his aesthetic inspiration. Therefore, careful consideration is needed for successful breast reconstruction to achieve optimal breast aesthetics, such as the size, shape, and placement of the breast mound [4].
In the Fourth Industrial Revolution era, there was a need to depend on the surgeon’s aesthetic inspiration for deciding the intraoperative breast volume expectation. Recently, more objective approaches for deciding breast volume have been studied. However, there was difficulty reaching a consensus on establishing a reliable method to achieve an aesthetically attractive breast until recently [56]. Many interactive factors define the final breast implant volume, such as implant type, implant placement plane, individual breast shape, and texture, all of which can affect the final reconstructed breast volume. Although the objective approach is not simple and perfectly correct, we are certain that this is the time to suggest a minimal range of objective breast volume expectations. Breast volume is considered an important factor in breast reconstruction; therefore, objective volumetry had to be developed.
A trial of the optimal analysis was conducted. Preoperatively, it is important to record anthropometric evaluations of normal and non-surgical breasts, which will be the definite final breast reconstruction goal and baseline information. The anthropomorphic method, thermoplastic cast, and Archimedean methods (water basin and cylinders) have been used to develop a technique for breast volume measurement [7]. In this study, a more objective evaluation was added through MRI of the breast to the existing basic numerical information. MRI is currently the primary diagnostic modality for breast cancer; thus, even without additional imaging evaluation, this information can be used to assess the actual breast volume [8]. Breast volume is achieved by the 3-dimensional (3D) concept of breast anthropometry; thus, through recent technology, multilateral efforts can be used to achieve breast volume.
This study aimed to conduct an MRI-based breast volumetric analysis. In addition, with periodic analysis of the 2-stage breast reconstruction in breast cancer patients, we suggest the possibility of clinical use of breast volumetry in final implant volume prediction.
This retrospective study included 140 patients who underwent unilateral 2-stage prosthetic breast reconstruction (tissue expander followed by permanent implant insertion) between January 1, 2017 and December 31, 2019. Two-stage breast reconstruction was defined as an immediate placement of a tissue expander after mastectomy (stage 1) followed by a change to a permanent implant after expansion postoperatively (stage 2) [3]. Patients who underwent 1-stage prosthetic breast reconstruction (direct implant insertion) or autologous tissue-based breast reconstruction were excluded.
Data were obtained from the electronic medical records. Data on age, height, weight, body mass index (BMI), smoking status, cancer profile and stage, volume and weight of mastectomy specimen, expander volume at initial and final follow-up, history of chemotherapy, radiotherapy, and hormone therapy were collected. Chemotherapy and radiotherapy were both further classified as neoadjuvant and adjuvant; data were recorded accordingly.
This study was approved by the Institutional Review Board of Ajou University Hospital (No. MED-MDB-21-235) and was performed in accordance with the tenets of the Declaration of Helsinki. This study was performed retrospectively, and the need for informed consent was waived. Nevertheless, consent to obtain and publish associated images was obtained from all patients, and the contents were included in the consent form for the surgery.
Immediate breast reconstruction was performed in all the patients after mastectomy, sentinel lymph node biopsy, or axillary lymph node dissection. In several cases, lymph node surgery was not performed in patients who had already undergone this procedure prior to the immediate breast reconstruction surgery. The weight of the mastectomy specimen was measured using an electronic scale. The breast volume was measured using the water displacement method. A cylinder was filled with one liter of normal saline, and the difference in volume was measured after inserting the specimen.
In the first stage, the tissue expander was placed on a subpectoral or prepectoral plane. The acellular dermal matrix (CG-CRYODERM, CGBio Corp., Seongnam, Korea) was used to cover the inferolateral surface of the prosthesis in the subpectoral plane, along with the entire surface of the prepectoral plane [9]. The size of the acellular dermal matrix used ranged from 60 to 324 cm2.
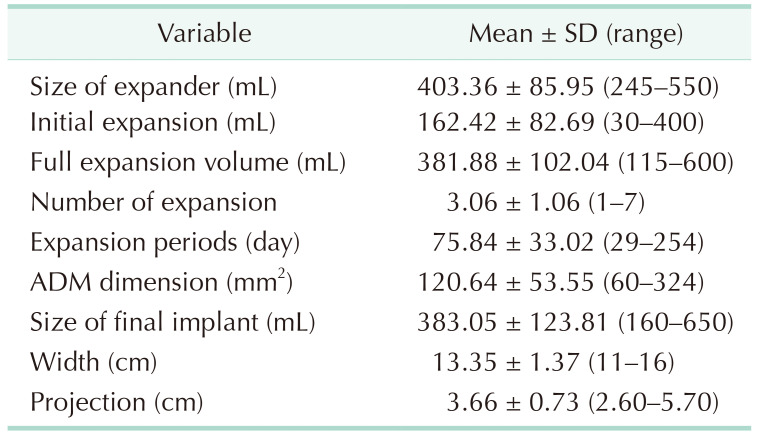
After the insertion of the expander and skin closure, an initial expansion was performed. Initial expansion volume was below 30%–50% of the expander size and was decided according to the skin elasticity by the surgeon’s evaluation to avoid provoking mastectomy flap necrosis. Thereafter, serial expansion was performed 2–4 weeks after the surgery [10]. The expansion interval was 2 weeks; however, it was adjusted according to the status of the skin flap. The total expansion period was counted as days, and the number of expansions was recorded.
In the 2nd stage of the surgery, tissue expander removal was performed with capsulotomy or capsulectomy. Subsequently, a permanent silicone gel implant was inserted. The final implant size was recorded and presented as milliliter (mL). Furthermore, the 2nd stage of the surgery was performed for at least 6 months after the completion of radiation therapy to allow soft tissue healing from radiation injury in patients who received radiation therapy.
MRI examinations were performed through a 3.0 T or 1.5 T whole-body scanner (Discovery 705 W 3T and sigma HDxt 1.5T, GE Health Care, Milwaukee, WI, USA). The patients adopted the prone position while wearing a premolded breast frame that prevents the breast from pressing downward. Avoiding downward pressure on the breasts is important for the accurate evaluation of their natural shape and volume. An MRI image was obtained before the breast cancer surgery and then regularly at 1, 3, 6, 12, and 24 months after the surgery. However, based on the cancer treatment status, data from either 3 months or 6 months after surgery were not recorded. In addition, the breast surgeon decided to perform MRI between 3 and 6 months according to the patient’s state.
First, data were available digitally and obtained from the PACS (picture archiving and communication system) system, and all slide images were uploaded to a 3D image reconstruction program (A-VIEW, Coreline Soft, Seoul, Korea). Second, the breast area was automatically calculated using artificial intelligence (AI)-based program protocol. Each axial image of the breast tissue boundary was manually revised by drawing on the program using only one conductor. In this study, breast mound was defined as outside breast on the dorsal aspect of the pectoral muscle [11]. The lateral border was defined as the point of the lateral thorax wall where the subcutaneous fat of the breast reached the same height as the subcutaneous fat of the thorax wall [7]. The dorsal boundary was determined as the parallel line of chest wall curvature in front of the rib and the lateral boundary as the point at which the subcutaneous fat of the breast was at the same level as the subcutaneous fat of the thorax wall [1213] (Fig. 1A, B). Third, each image was automatically integrated into a 3-image format after manual revision (Fig. 1C, D). The volume of the marked area was calculated and presented in mL.
R language ver. 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria) and the T&F program ver. 3.0 (YooJin BioSoft, Korea) was used for all the statistical analyses. Breast volumes measured preoperatively and postoperatively are presented as medians (interquartile ranges). The Wilcoxon signed-rank test was performed for the mean comparison of breast volumes between the preoperative and postoperative time points. The Bonferroni method was used for multiple comparisons.
The postoperative volume changes from the final implant size were computed and analyzed using the Kruskal-Wallis H-test. Univariable linear regression analysis was performed to extract linear equations for predicting the final implant size using the breast volume measured at each postoperative time as the independent variable. A significance level of P < 0.05 was used for all analyses.
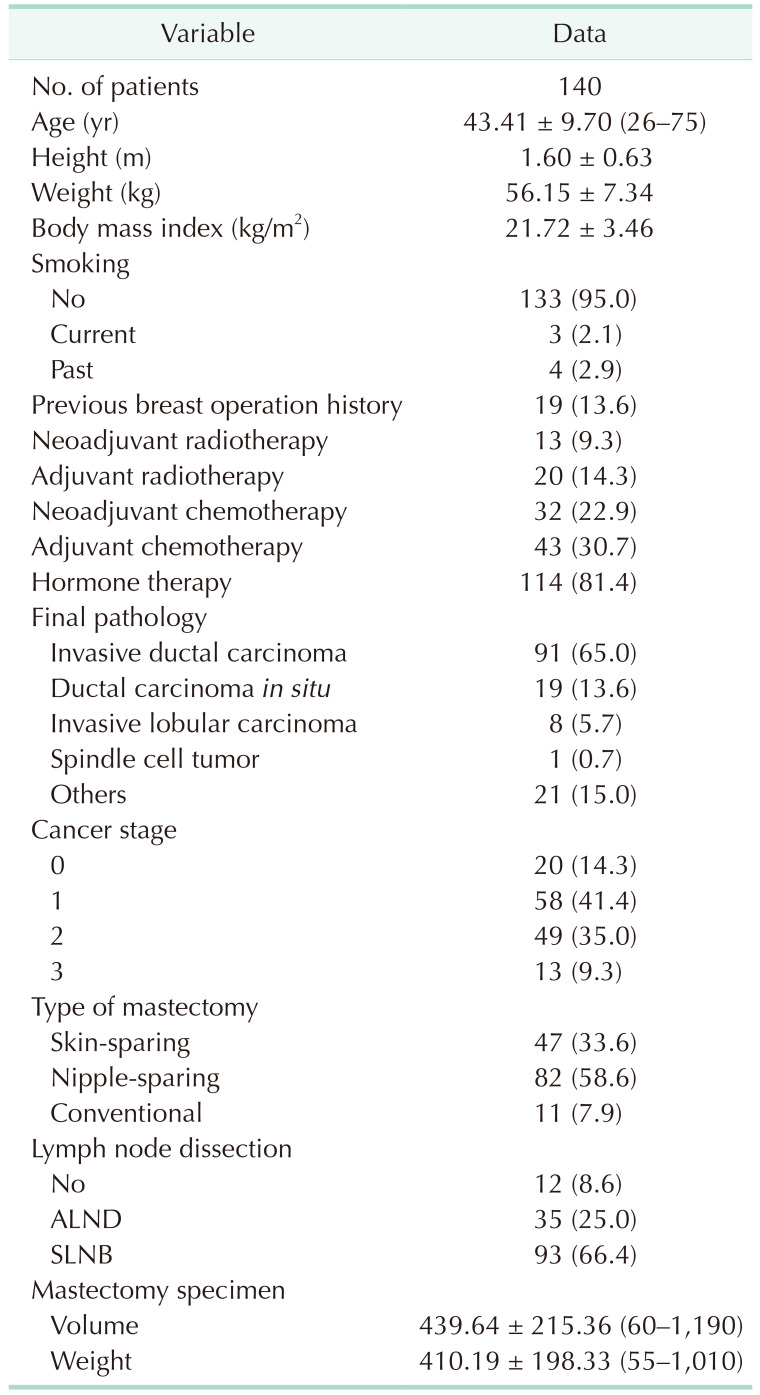
A total of 140 patients with a mean age of 43.41 years (standard deviation, ±9.70) were evaluated. The mean height and weight were 1.60 m (±0.63) and 56.15 kg (±7.34), respectively. The mean BMI was 21.72 kg/m2 (±3.46). Details of the chemotherapy and radiotherapy history are described in Table 1. The mean volume of the mastectomy specimen was 439.64 mL (±215.36), and the mean weight was 410.19 g (±198.33). Immediate tissue expander insertion was performed after mastectomy in all patients. The details of the expansion strategy are described in Table 2. The mean expander was 403.36 mL (±85.95). The mean number of expansions was 3.06 (±1.06), and the mean expansion period was 75.84 days (±33.02). After expansion, the mean full expansion volume was 381.88 mL (±102.04), and the mean final implant was 383.05 mL (±123.81).
Due to the retrospective nature of this study, we collected the precaptured MRI data. Unfortunately, we missed some specimens during follow-up, particularly between 3 and 6 months of follow-up. Thus, we conducted statistical evaluation only on the obtainable data of 114 patients.
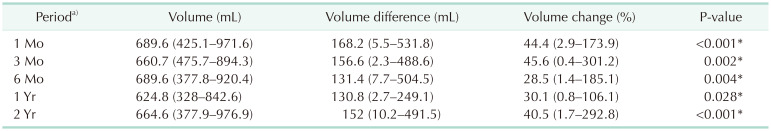
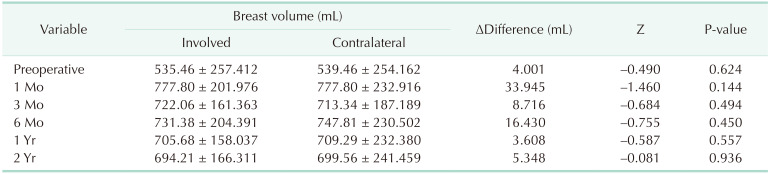
We calculated the mean volume measurements for each period and analyzed the change in periodical volume difference (Table 3). Regarding breast cancer-involved site volume, a statistically significant difference was identified in all postoperative periods. At 1 month postoperatively, maximized breast volume and volume differences were noted and compared with the preoperative volume. The volume at 1 month was 689.6 mL (range, 425.1–971.6 mL), and the difference was 168.2 mL (range, 5.5–531.8 mL) (P < 0.001). The postoperative volume at 1 year was minimized, the volume was 624.8 mL (range, 328.0–842.6 mL) and the difference was 130.8 mL (range, 2.7–249.1 mL) (P = 0.028). Fig. 2 illustrates these periodical trends. The breast volume and BMI were maximized and minimized at 1 month and 1 year postoperatively, respectively (Fig. 2). There were 2 downward trends in 1–3 months and 6 months–1 year, but the minimized volume was checked in 1 year and maximized volume checked in 1 month. Fig. 2 also shows BMI change trends. Interestingly, after completion of mastectomy, 80.9% of patients underwent hormonal therapy (Table 1) with oral medications, and this was associated with an increase in BMI. The relationship between the cancer-involved site volume and BMI is plotted in Fig. 2.
In the cancer-involved breast site, the periodic trend of the breast volume difference between the MRI-based calculated breast volume and actual inserted implant volume was evaluated and presented in Fig. 3. The X-axis shows the postoperative period from the expander-to-implant change operation, while the Y-axis shows volume changes between the postoperative volume and actual implant volume. The median breast volume at 1 year postoperatively showed a minimal volume difference (279.0 mL [interquartile range, 232.8–386.3 mL], P < 0.001). From this result, we identified that the postoperative volume at 1 year had a significant influence on volume estimation (Fig. 3, Supplementary Table 1).
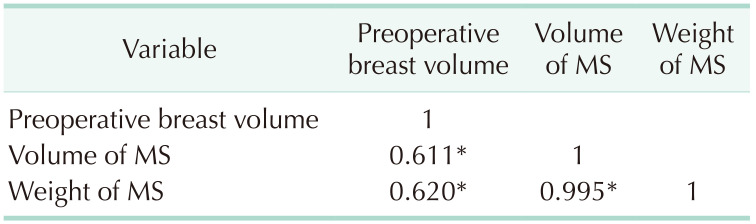
For the validation of MRI volumetry reliability, the correlation between the MRI-based preoperative breast volumetry and mastectomy specimen volume was determined. Specimen weight was also evaluated. A positive correlation was observed between preoperative volume and each mastectomy specimen. Volume and weight were 0.611 and 0.620, respectively (P < 0.01). Data of breast volumetry showed statistical significance (Table 4).
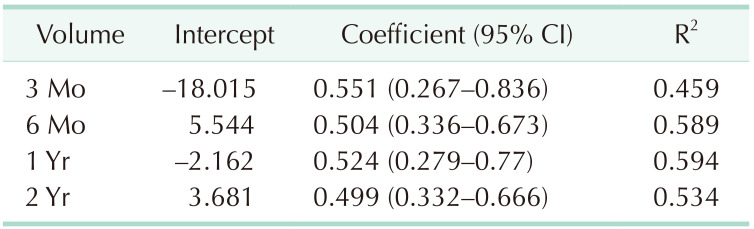
We used a univariable linear regression test to investigate the relationship between final implant size and postoperative breast volume. In the linear regression test, the P-value was used as a measure of the reliability of the values of intercept and coefficient. Table 5 shows all statistically significant values. The R2 coefficient of determination indicates the power of each period volume to explain the final implant size. Among all the periods, the volume at 1 year had the highest R2 value and a powerful effect on the final implant size (P < 0.001, R2 = 0.594) (Table 5). The relationship between the final implant size and the postoperative breast volume can be described through the formula described below.
Moreover, the equation of postoperative 1 year with the closest volume of the final implant size was also acquired and expressed above and was presented as a graph (Fig. 4).
Bilateral breast volume symmetry was evaluated, which correlated with an aesthetic factor of the breast. There was no statistically significant difference between the cancer-involved breast and the contralateral breast in each period. From this result, it was observed that both breasts were similarly maintained during all periods (Table 6).
Due to the aesthetic importance of breast volume, several trials have been conducted to determine actual breast volume [1415]. Early trials were very direct measurements of the volume. In the earliest report of breast volume measurement by Bouman, various techniques were used, such as the water volume displacement method, negative molding using thermoplastic casts, and direct anthropomorphic measurements [1617]. However, stereoscopic anatomical characteristics of the breast are limited by direct approaches. Recent studies have focused on accurate anthropomorphic measurement using 2-dimensional (2D) imaging or 3D imaging and 3D surface scanning technology and have been described with varying accuracy and reliability. In particular, 3D surface imaging is regarded as the most promising method in volumetric analysis and thus, has been extensively studied [18]. This technology has been adopted and developed in many fields; however, due to the complexity of breast shape, this method may not easily find use in breast reconstruction due to inaccuracy [18], which is of paramount importance in achieving actual breast volume.
Due to the current inaccuracy of breast volume determination, in this article we propose a combination method of MRI and AI-based breast volumetry. Several authors have reported that combining this with conventional 3D imaging modalities, such as mammography, CT, and MRI, enables visualization of the internal structures, potentially leading to more accurate estimation of the breast parenchymal volume [1819]. To date, most CT scans used for breast volume measurement are 3D. In this study, we suggest a new method of adopting MRI imaging. Different from CT scan-based measurements, MRI-based methods require more technically difficult 3D reconstruction. However, the application of an AI-based program protocol in our study system abrogates this problem. Once all slide images are uploaded, the program initially automatically performs breast imaging, which is then used to reconfirm the breast boundary landmarks. This system is different from other conventional breast volumetry methods. MRI volumetry has several strengths compared with CT-based imaging volumetry. MRI is a useful tool that allows regular follow-up of patients with breast cancer. The CT software manually or automatically creates a region of interest (ROI) by segmenting individual axial slices based on imaging data. However, MRI has more narrowing ROI and is a more validated imaging modality compared with CT. Rha et al. [20] reported that in a comparison of volume measurement between plaster cast and MRI, 3D simulated MRI showed more accuracy (mean error: plaster cast, 137.4 ± 97.66 mL vs. MRI, 54.63 ± 46.30 mL) than plaster cast. Kim et al. [21] compared the modalities and demonstrated that MRI is more accurate than CT in determining the volume of resected breast tissue (0.928 vs. 0.782, P = 0.001). Fowler et al. [22] reported a deviation error of only 4.3% when the breast volume was measured by MRI.
Furthermore, MRI allows position consideration during evaluation and is thus a better modality for breast volumetry. The breasts comprise soft tissue; thus, their shape and volume can change according to the individual’s posture. To accurately determine the breasts’ natural shape and volume, it is important that they remain free from any compressive pressure. During surgery, patients lay supine on the operating table; however, this position limits accurate breast shape determination, because the breasts are prone to sagging laterally due to gravity. Therefore, many plastic surgeons use special operation-table settings that enable patients to assume a sitting position intraoperatively. Thus, the patient’s position during surgery is an important factor to consider in breast volumetry. MRI has a prone-position setting that allows patients to lie with their breasts hanging free in the recesses of the coil. This design allows the breast tissue to spread, which facilitates detection of abnormalities and prevents motion artifacts induced by respiration [23]. Therefore, MRI-based volumetry is the most appropriate modality for measuring breast volume, irrespective of the patient’s position.
This study raises several important clinical questions. First, is there a way to predict the final implant size during the preoperative period? If we answer this, it will be a very helpful guide during the selection of intraoperatively appropriate implant sizes. Choosing an appropriate breast implant, in terms of volume, is a critical step in well-performed breast augmentation and reduction mammoplasty [24]. Furthermore, in implant-based breast aesthetics, size is arguably the most critical aspect of implant selection, followed by shape, profile, and surface textures as other considerable factors [6]. Accurate implant selection prior to 2-stage breast reconstruction is essential for good postoperative results in symmetrical breasts, especially in locations with restricted access to symmetrizing surgery [25]. Thus, we can reach a wider application in clinical practice, which is a great advancement in the field of breast surgery. Hopefully, this will begin a new era of implant selection based on MRI breast volumetric information.
The second question is how can we minimize the gap between recent volumetry technology and operative field in breast reconstruction surgery? To answer this question, we suggest using the formula from our study results. In the expander-to-implant 2-stage breast reconstruction, patients had to undergo a long period of radiation treatment. Radiation causes breast skin envelope contracture and creates a different texture. Even though in the first step of the surgery, the surgeon estimated the mastectomy volume and attempted to insert the most symmetrical breast expander volume on the contralateral side, after the radiotherapy period, the patient positioned in the second operating room was completely different from the one on which the first surgery was carried out. Regarding this discrepancy, we suggest the determination of postoperative timing in how to use this formula practically. Using an MRI-based 3D breast volume calculation, breast volume was maximized at 1 month postoperatively, and volume differences were noted and compared to the preoperative volume. We found that the postoperative volume at 1 year was minimized in this study.
Third, what will be the best judgment of intraoperative implant size selection? Here, this study aimed to provide proper final breast implant volume estimates based on preoperative breast volume mastectomy. It is true that volume match alone is insufficient to define a desirable outcome in breast reconstruction. A good volume match can achieve a good cosmetic result; however, there are many contributing factors to successful breast reconstruction. Table 1 shows the many factors of breast cancer patients that should be considered in intraoperative decision-making. However, despite the complexity, modern society wants faster results, and the technological development that results from them makes it possible. Computerized numerical data from preoperative breast MRI volumetry, interoperative mastectomy volume information, periodic volume change through radiation, and finally inserted breast implant stabilization and periodical change could sufficiently introduce this estimation formula. Among all the periods, the volume at 1 year had the highest R2 value and a powerful effect on the final implant size. The main goal of the reconstructive surgeon is to achieve symmetry in the final reconstruction stage. Thus, once the final implant volume can be estimated in the 2nd-stage surgery, the operative 1-year volume can be easily estimated using our results. Depending on the patient’s preference, the surgeon and patient’s estimates can be adjusted, and a decision made for implant size intraoperatively with preoperative consultation. In our center, especially in Asian patients, we intend to make the 1-year breast volume match the preoperative breast volume.
Regarding the minimized breast volume at 1 year, and the fact that this time was optimal in matching intraoperative implant size, some possible reasons can be deduced. At 1 year, the average breast volume was 624.8 mL and the volume difference was 130.8 mL. This periodical volume change may initially be caused by postoperative edema, which would explain the maximal breast volume at 1 month. This begins to decrease from 3 to 6 months, which could be explained by decreased generalized edema and repositioning of the implant. BMI may also be a possible cause of periodical change. In Fig. 2, we correlated BMI changes with breast volume changes. We found that BMI was maximized at 1 month and minimized at 1 year. A previous study also showed that breast volume is affected by BMI [26], which can be affected by various factors such as chemotherapy, radiotherapy, and hormone therapy. Hormone therapy was offered on the basis of the results of a hormone receptor test even to patients with ductal carcinomas in situ; the decision to administer therapy was left to the breast surgeon’s discretion. From our study results, postoperative 1-year breast volume was the best matching volume with the preoperative breast volume.
We believe that our methodology using MRI breast volumetry is very promising. Breast area has always been a challenge to determine with computer technology, and this study considers the causes for this. The advent of a practical method for objective assessment poses an emerging benefit to the field of reconstructive breast surgery. The possible clinical benefits of advancement in imaging can provide invaluable tools for reverse engineering, digital archiving, quality inspection, and animation. In addition to breast surgery, dentistry, craniofacial surgery, and aesthetic facial surgery have adopted recent technological advancements [21272829]. However, the adoption of such advanced technology for breast surgery has not been finalized and is still in the early stages of the revolution. Therefore, we think it is time to adopt this knowledge in actual clinical settings, which we performed with 3D breast volumetry [24]. In addition, there are reports that the information from 3D volumetric data helps surgeons guide tissue expansion and surgical management. Therefore, the provision of clinically relevant data can surely provide an impactful aid in reconstruction [24].
This study had some limitations. First, the study outcome was limited by inherent selection bias, misclassification, or information bias due to its retrospective nature. Some key statistical measures that can affect outcome assessment could not be measured. Second, many reports showed that mastectomy volume and weight varied according to the surgeon’s factor. Mastectomy specimen and weight are strongly dependent on the breast surgeon’s preference, surgical characteristics, career, and academic pursuits. Even at the same cancer center, different surgeons have different mastectomy results. Hence, our study formula results might not be adapted conventionally for other breast cancer patients. As this is a single-center study, the interpretation can differ depending on the amount of residual subcutaneous fat, it because the remaining tissue dependent to breast surgeon and there are many proven results that has a wide range of surgeons’ preference. However, regarding the discrepancy between soft tissue volume of the breasts and actual breast volume, we believe our MRI-based method can minimize this gap. There are very few studies of breast volumetry using MRI and AI concurrently. In the methodology, only a few steps are required to obtain breast volume. Using this accumulated retrospective breast volume data can help to estimate the best matching breast implant volume. In future studies, we plan to prospectively design studies and aim to use our formula to allow the surgeon to decide on implant size first, and then compare lateral volume. Third, our study evaluated only breast volume data. Volume match is one of the important factors in evaluating cosmetic breast reconstruction results. In this study, successful breast reconstruction was defined as good volume harmony; however, this definition is debated. Fourth, this study used MRI information obtained only for cancer follow-up. We did not perform additional imaging for the breast volume follow-up. Therefore, periodic data could be missing depending on the patient’s cancer status. Insufficient data were excluded in this study; however, different follow-up MRI schedules resulted in a selection bias.
We demonstrated the clinical usefulness of the latest technology using MRI-based breast volumetry, especially in 2-stage breast reconstruction, in which perioperative breast volume prediction has a powerful meaning. The combination of manual landmark confirmation and AI-based automatic 3D image regeneration ensured the accuracy of this method. Based on this study, MRI-based breast volumetry could be a tool for reconstruction surgeons to inspect preoperative planning more objectively. In this study, we suggested a volume prediction formula that describes the relationship between postoperative breast volume and the final breast implant size. We believe that our study can be a stepping stone in an era of more scientific and objective breast reconstruction surgery.
Notes
Fund/Grant Support: This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant No., HI20C2140). This work was also supported by the intramural research fund of Ajou University Medical Center.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. PMID: 33538338.

2. Stevens LA, McGrath MH, Druss RG, Kister SJ, Gump FE, Forde KA. The psychological impact of immediate breast reconstruction for women with early breast cancer. Plast Reconstr Surg. 1984; 73:619–628. PMID: 6709743.

3. Lam TC, Hsieh F, Boyages J. The effects of postmastectomy adjuvant radiotherapy on immediate two-stage prosthetic breast reconstruction: a systematic review. Plast Reconstr Surg. 2013; 132:511–518. PMID: 23676964.

4. Yip JM, Watson DI, Tiggemann M, Hsia S, Smallman AE, Dean NR. Determinants of breast reconstruction outcome: how important is volume symmetry? J Plast Reconstr Aesthet Surg. 2015; 68:679–685. PMID: 25731778.

5. Blondeel PN, Hijjawi J, Depypere H, Roche N, Van Landuyt K. Shaping the breast in aesthetic and reconstructive breast surgery: an easy three-step principle. Plast Reconstr Surg. 2009; 123:455–462. PMID: 19182601.

7. Koch MC, Adamietz B, Jud SM, Fasching PA, Haeberle L, Karbacher S, et al. Breast volumetry using a three-dimensional surface assessment technique. Aesthetic Plast Surg. 2011; 35:847–855. PMID: 21487916.

8. Herold C, Reichelt A, Stieglitz LH, Dettmer S, Knobloch K, Lotz J, et al. MRI-based breast volumetry-evaluation of three different software solutions. J Digit Imaging. 2010; 23:603–610. PMID: 20066465.

9. Vidya R, Masià J, Cawthorn S, Berna G, Bozza F, Gardetto A, et al. Evaluation of the effectiveness of the prepectoral breast reconstruction with Braxon dermal matrix: first multicenter European report on 100 cases. Breast J. 2017; 23:670–676. PMID: 28481477.

10. Burkhardt BR, Eades E. The effect of Biocell texturing and povidone-iodine irrigation on capsular contracture around saline-inflatable breast implants. Plast Reconstr Surg. 1995; 96:1317–1325. PMID: 7480228.

11. Kovacs , Eder M, Hollweck , Zimmermann A, Settles M, Schneider A, et al. Comparison between breast volume measurement using 3D surface imaging and classical techniques. Breast. 2007; 16:137–145. PMID: 17029808.

12. Yoo A, Minn KW, Jin US. Magnetic resonance imaging-based volumetric analysis and its relationship to actual breast weight. Arch Plast Surg. 2013; 40:203–208. PMID: 23730594.

13. Kovacs , Eder M, Hollweck , Zimmermann A, Settles M, Schneider A, et al. New aspects of breast volume measurement using 3-dimensional surface imaging. Ann Plast Surg. 2006; 57:602–610. PMID: 17122543.

14. Hudson DA. Factors determining shape and symmetry in immediate breast reconstruction. Ann Plast Surg. 2004; 52:15–21. PMID: 14676693.

15. Lee HY, Hong K, Kim EA. Measurement protocol of women’s nude breasts using a 3D scanning technique. Appl Ergon. 2004; 35:353–359. PMID: 15159200.

16. Kayar R, Civelek S, Cobanoglu M, Gungor O, Catal H, Emiroglu M. Five methods of breast volume measurement: a comparative study of measurements of specimen volume in 30 mastectomy cases. Breast Cancer (Auckl). 2011; 5:43–52. PMID: 21494401.

17. Bulstrode N, Bellamy E, Shrotria S. Breast volume assessment: comparing five different techniques. Breast. 2001; 10:117–123. PMID: 14965570.

18. Chae MP, Rozen WM, Spychal RT, Hunter-Smith DJ. Breast volumetric analysis for aesthetic planning in breast reconstruction: a literature review of techniques. Gland Surg. 2016; 5:212–226. PMID: 27047788.
19. Kim JS, Bae K, Lee EJ, Bang M. Mammography with a fully automated breast volumetric software as a novel method for estimating the preoperative breast volume prior to mastectomy. Ann Surg Treat Res. 2021; 100:313–319. PMID: 34136427.

20. Rha EY, Choi IK, Yoo G. Accuracy of the method for estimating breast volume on three-dimensional simulated magnetic resonance imaging scans in breast reconstruction. Plast Reconstr Surg. 2014; 133:14–20.

21. Kim H, Mun GH, Wiraatmadja ES, Lim SY, Pyon JK, Oh KS, et al. Preoperative magnetic resonance imaging-based breast volumetry for immediate breast reconstruction. Aesthetic Plast Surg. 2015; 39:369–376. PMID: 25924697.

22. Fowler PA, Casey CE, Cameron GG, Foster MA, Knight CH. Cyclic changes in composition and volume of the breast during the menstrual cycle, measured by magnetic resonance imaging. Br J Obstet Gynaecol. 1990; 97:595–602. PMID: 2390502.

23. Konyer NB, Ramsay EA, Bronskill MJ, Plewes DB. Comparison of MR imaging breast coils. Radiology. 2002; 222:830–834. PMID: 11867809.

24. Tepper OM, Karp NS, Small K, Unger J, Rudolph L, Pritchard A, et al. Three-dimensional imaging provides valuable clinical data to aid in unilateral tissue expander-implant breast reconstruction. Breast J. 2008; 14:543–550. PMID: 19054001.

25. Szychta P, Raine C, Butterworth M, Stewart K, Witmanowski H, Zadrozny M, et al. Preoperative implant selection for two stage breast reconstruction with 3D imaging. Comput Biol Med. 2014; 44:136–143. PMID: 24377697.

26. Coltman CE, Steele JR, McGhee DE. Breast volume is affected by body mass index but not age. Ergonomics. 2017; 60:1576–1585. PMID: 28532249.

27. Lee S. Three-dimensional photography and its application to facial plastic surgery. Arch Facial Plast Surg. 2004; 6:410–414. PMID: 15545536.

28. Ciocca L, Scotti R. CAD-CAM generated ear cast by means of a laser scanner and rapid prototyping machine. J Prosthet Dent. 2004; 92:591–595. PMID: 15583570.

29. Avis NJ, Briggs NM, Kleinermann F, Hose DR, Brown BH, Edwards MH. Anatomical and physiological models for surgical simulation. Stud Health Technol Inform. 1999; 62:23–29. PMID: 10538363.
SUPPLEMENTARY MATERIALS
Supplementary Table 1 can be found via https://doi.org/10.4174/astr.2022.103.4.195.
Supplementary Table 1
Statistical analysis of perioperative breast volumetry in final implant size prediction
Fig. 1
MRI-based three-dimensional (3D) reconstructed breast image. Using an artificial intelligence-based 3D image reconstruction program (A-VIEW, Coreline Soft, Seoul, Korea), the breast tissue boundary was drawn automatically and manually revised using only one conductor (purple area). (A, B) The dorsal boundary was set as a parallel line of the chest wall curvature in front of the rib. The lateral border was defined as the point at which the subcutaneous fat of the breast was located at the same level as the subcutaneous fat of the thorax wall. After revision, each image was automatically integrated into a 3D image. (C, D) Extracted breast volume.

Fig. 2
Periodical change of cancer-involved breast site and three-dimensional volumetry in 2-stage breast reconstruction compared to preoperative volume. The solid line indicates the breast cancer site volume, while the dotted line indicates body mass index (BMI). The relationship between the cancer-involved site volume and BMI was plotted in a single figure. Breast volume and BMI were maximized and minimized at 1 month and 1 year postoperatively, respectively.

Fig. 3
Periodical trend of breast volume difference between MRI-based preoperative state and actual implant volume. Box plot represents the median (interquartile range) postoperative breast volume change compared to the final implant size. The mean breast volume change is represented by a black line. The median breast volume at 1 year postoperatively showed a minimal volume difference (P < 0.001).
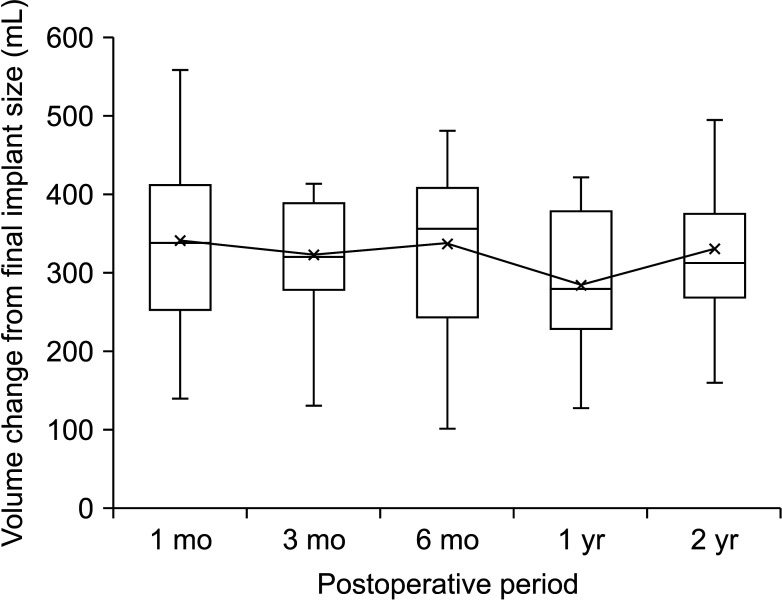
Fig. 4
Univariable linear regression using final implant size and postoperative breast volume. The relationship between the final implant size and the postoperative breast volume at 1 year is represented as a distributed chart. The solid line indicates the equation of 1-year postoperative breast volume according to the final implant size.
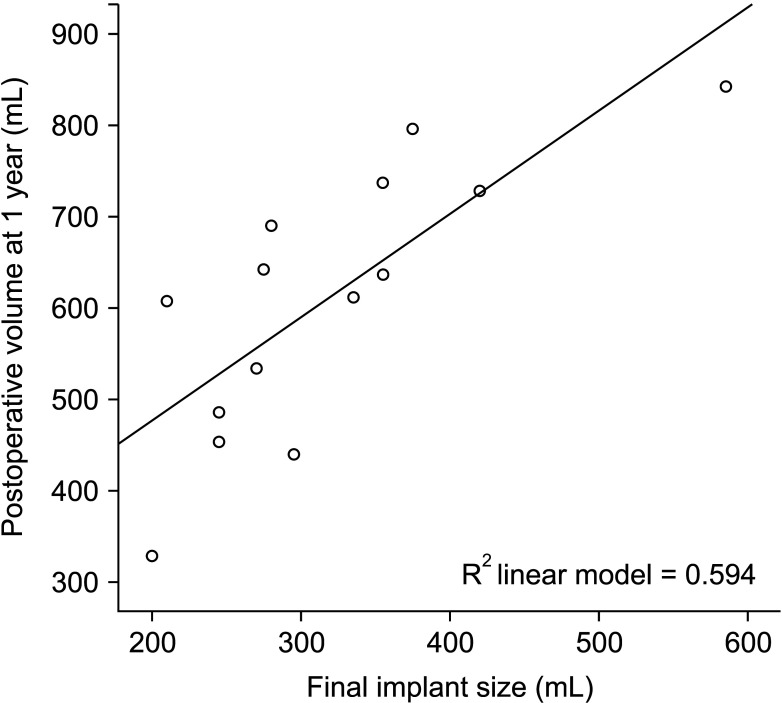
Table 3
Periodic changes in cancer-involved breast site three-dimensional volumetry in 2-stage breast reconstruction compared to preoperative volume




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download