1. Hoorweg BB, Willemsen RT, Cleef LE, et al. Frequency of chest pain in primary care, diagnostic tests performed and final diagnoses. Heart. 2017; 103:1727–1732. PMID:
28634285.

2. Roifman I, Sivaswamy A, Chu A, et al. Clinical effectiveness of cardiac noninvasive diagnostic testing in outpatients evaluated for stable coronary artery disease. J Am Heart Assoc. 2020; 9:e015724. PMID:
32605412.

3. Virani SS, Alonso A, Benjamin EJ, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020; 141:e139–e596. PMID:
31992061.
4. Task Force Members. Montalescot G, Sechtem U, et al. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013; 34:2949–3003. PMID:
23996286.

5. Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2012; 60:e44–164. PMID:
23182125.
6. Wolk MJ, Bailey SR, Doherty JU, et al. ACCF/AHA/ASE/ASNC/HFSA/HRS/SCAI/SCCT/SCMR/STS 2013 multimodality appropriate use criteria for the detection and risk assessment of stable ischemic heart disease: a report of the American college of cardiology foundation appropriate use criteria task force, American heart association, American society of echocardiography, American society of nuclear cardiology, heart failure society of America, heart rhythm society, society for cardiovascular angiography and interventions, society of cardiovascular computed tomography, society for cardiovascular magnetic resonance, and society of thoracic surgeons. J Am Coll Cardiol. 2014; 63:380–406. PMID:
24355759.
7. Keyhani S, Falk R, Bishop T, Howell E, Korenstein D. The relationship between geographic variations and overuse of healthcare services: a systematic review. Med Care. 2012; 50:257–261. PMID:
22329997.

8. Chinnaiyan KM, Raff GL, Goraya T, et al. Coronary computed tomography angiography after stress testing: results from a multicenter, statewide registry, ACIC (Advanced Cardiovascular Imaging Consortium). J Am Coll Cardiol. 2012; 59:688–695. PMID:
22322086.
9. Patel MR, Peterson ED, Dai D, et al. Low diagnostic yield of elective coronary angiography. N Engl J Med. 2010; 362:886–895. PMID:
20220183.

10. Marwick TH, Cho I, Ó Hartaigh B, Min JK. Finding the gatekeeper to the cardiac catheterization laboratory: coronary CT angiography or stress testing? J Am Coll Cardiol. 2015; 65:2747–2756. PMID:
26112200.
11. Chang HJ, Lin FY, Gebow D, et al. Selective referral using CCTA versus direct referral for individuals referred to invasive coronary angiography for suspected CAD: a randomized, controlled, open-label trial. JACC Cardiovasc Imaging. 2019; 12:1303–1312. PMID:
30553687.

12. SCOT-HEART Investigators. Newby DE, Adamson PD, et al. Coronary CT angiography and 5-year risk of myocardial infarction. N Engl J Med. 2018; 379:924–933. PMID:
30145934.

13. Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. N Engl J Med. 2015; 372:1291–1300. PMID:
25773919.

14. Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005; 43:1130–1139. PMID:
16224307.

15. Kim L, Kim JA, Kim S. A guide for the utilization of health insurance review and assessment service national patient samples. Epidemiol Health. 2014; 36:e2014008. PMID:
25078381.

16. Reeves RA, Halpern EJ, Rao VM. Cardiac imaging trends from 2010 to 2019 in the Medicare population. Radiol Cardiothorac Imaging. 2021; 3:e210156. PMID:
34778785.

17. Morris JR, Bellolio MF, Sangaralingham LR, et al. Comparative trends and downstream outcomes of coronary computed tomography angiography and cardiac stress testing in emergency department patients with chest pain: an administrative claims analysis. Acad Emerg Med. 2016; 23:1022–1030. PMID:
27155236.

18. Levin DC, Parker L, Halpern EJ, Rao VM. Coronary CT angiography: use in patients with chest pain presenting to emergency departments. AJR Am J Roentgenol. 2018; 210:816–820. PMID:
29446681.

19. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008; 52:1724–1732. PMID:
19007693.

20. Dharampal AS, Papadopoulou SL, Rossi A, et al. Diagnostic performance of computed tomography coronary angiography to detect and exclude left main and/or three-vessel coronary artery disease. Eur Radiol. 2013; 23:2934–2943. PMID:
23812241.

21. Dewey M, Rief M, Martus P, et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: randomised controlled trial. BMJ. 2016; 355:i5441. PMID:
27777234.

22. Nielsen LH, Markenvard J, Jensen JM, Mickley H, Øvrehus KA, Nørgaard BL. Frontline diagnostic evaluation of patients suspected of angina by coronary computed tomography reduces downstream resource utilization when compared to conventional ischemia testing. Int J Cardiovasc Imaging. 2011; 27:813–823. PMID:
21042860.

23. Reynolds HR, Shaw LJ, Min JK, et al. Outcomes in the ISCHEMIA trial based on coronary artery disease and ischemia severity. Circulation. 2021; 144:1024–1038. PMID:
34496632.
24. Ferencik M, Mayrhofer T, Bittner DO, et al. Use of high-risk coronary atherosclerotic plaque detection for risk stratification of patients with stable chest pain: a secondary analysis of the PROMISE randomized clinical trial. JAMA Cardiol. 2018; 3:144–152. PMID:
29322167.

25. Xu J, Cai F, Geng C, Wang Z, Tang X. Diagnostic performance of CMR, SPECT, and PET imaging for the identification of coronary artery disease: a meta-analysis. Front Cardiovasc Med. 2021; 8:621389. PMID:
34026862.

26. Takx RA, Blomberg BA, El Aidi H, et al. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ Cardiovasc Imaging. 2015; 8:e002666. PMID:
25596143.

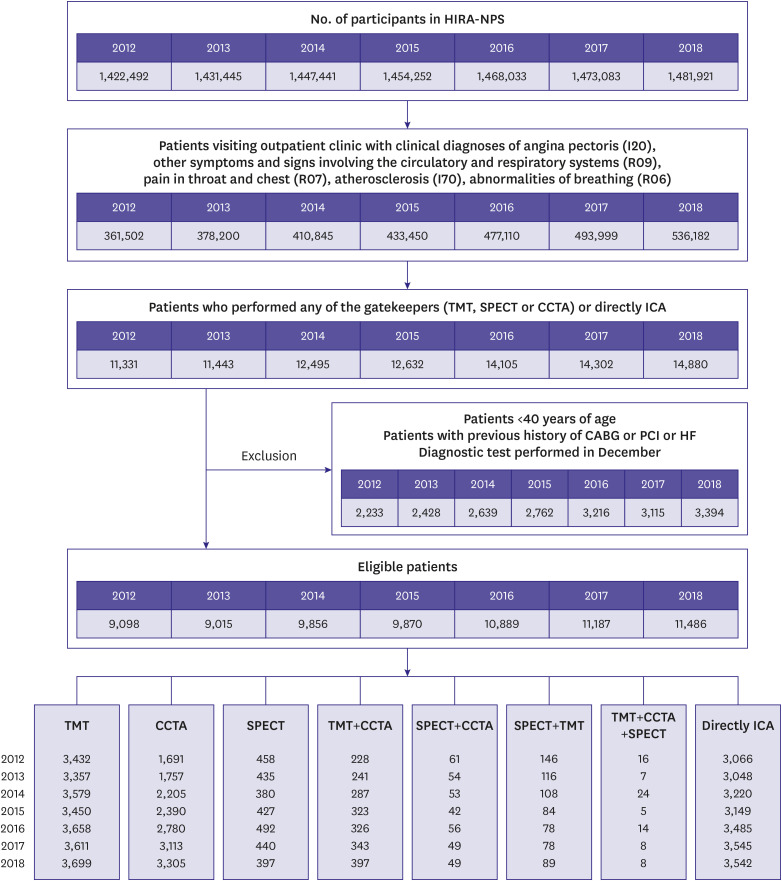
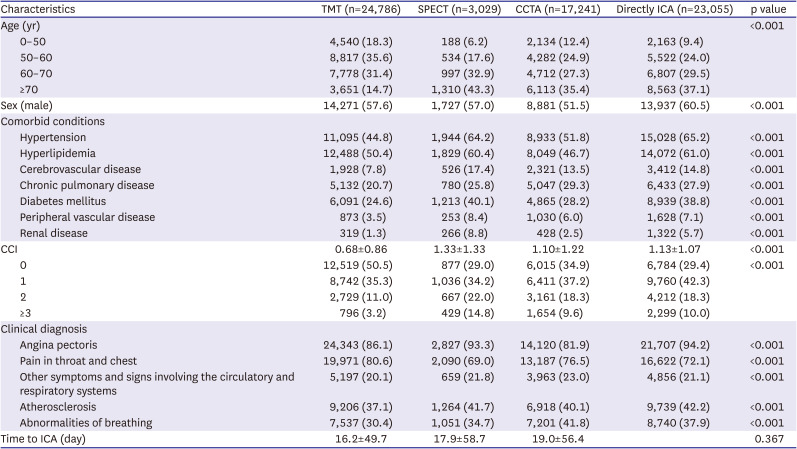

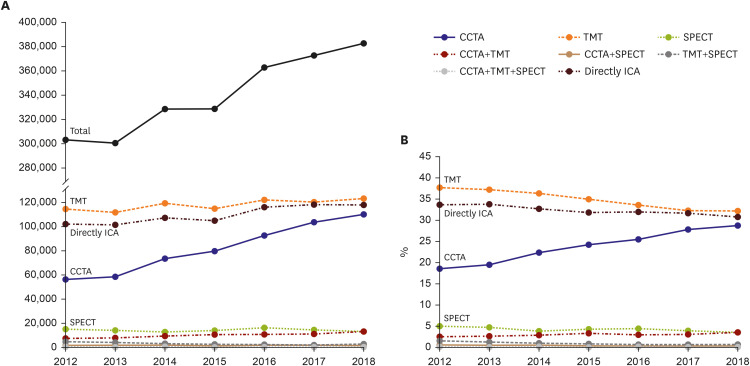
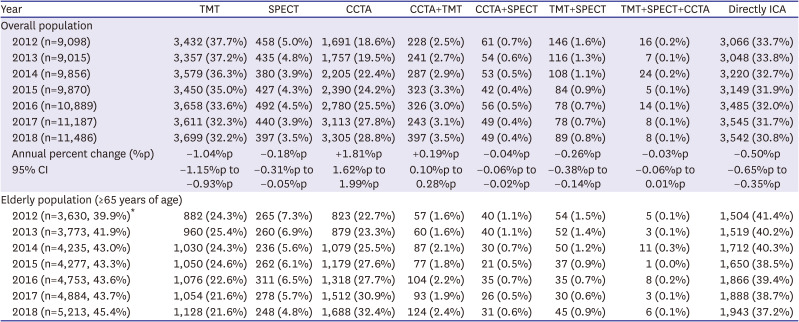





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download