Abstract
Papillary thyroid carcinoma is the most common type of thyroid cancer, for which surgery following preoperative staging and risk assessment is the standard treatment. Afatinib is an orally active irreversible ErbB-family inhibitor that binds to the kinase domain of epidermal growth factor receptors (EGFRs), HER2, and HER4, and has been approved as monotherapy for the treatment of locally advanced or metastatic non-small cell lung cancer with activated EGFR mutations. Recently, we observed an unexpected effect of afatinib administered to treat lung cancer on untreated papillary thyroid carcinoma.
Papillary thyroid carcinoma (PTC) is the most common histologic type of malignant tumor of the thyroid gland.1) Surgical management, involving either lobectomy or total thyroidectomy, is generally recommended after preoperative staging and risk assessment.2) Afatinib is an orally active, irreversible ErbB-family inhibitor that binds to the kinase domain of human epidermal growth factor receptors (EGFRs), including HER2 and HER4, which are used for targeted molecular therapy.3) Afatinib has been approved as monotherapy for the treatment of locally advanced or metastatic non-small cell lung cancer (NSCLC) with activated EGFR mutations.4)
Although several trials have been conducted to identify novel treatments for PTC by investigating the molecular pathways involved in its development,5) there are few studies about the effect of afatinib on PTC.
Here, we report an unexpected effect of afatinib administered for advanced lung cancer treatment, on papillary thyroid cancer.
Written informed consent was obtained from the patient for the publication of this case report.
A 55-year-old female patient presented with a diagnosis of double primary cancers in the thyroid and lung, as evaluated at a local hospital. The chief complaint was chest pain; no other abnormal findings were observed on physical examination.
Her thyroid function was within normal limits: T3 of 103.4 ng/dL (71-161 ng/dL), free T4 of 1.1 ng/dL (0.8-1.7 ng/dL), thyroid stimulating hormone (TSH) of 2.95 μIU/mL (0.86-4.69 μIU/mL), thyroglobulin antigen of 16.83 ng/dL (0.04-32.5 ng/dL), and anti-thyroglobulin antibody of 19.4 μIU/mL (10-124.2 μIU/mL).
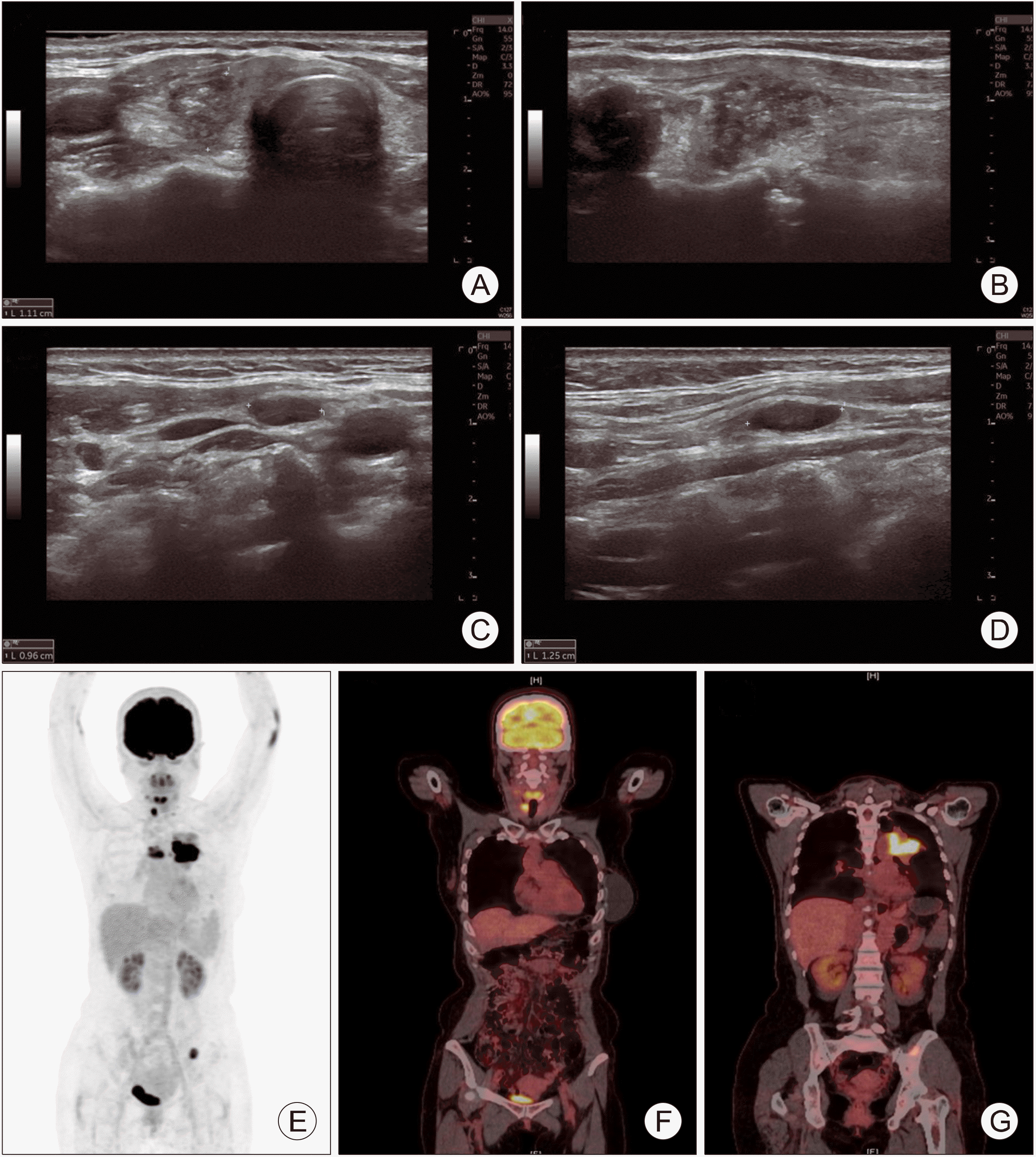
Imaging studies, including computed tomography (CT), ultrasonography (US), positron emission tomography (PET), and whole-body bone scan (WBBS) were performed (Fig. 1). Thyroid US and CT showed a 1.3-cm sized infiltrative enhancing lesion in the right thyroid gland and suspicious lymph nodes in the right lateral neck (levels III and IV). A primary lung cancer lesion in the left upper lobe was observed on chest CT, with multiple metastatic lymph nodes in the mediastinum. PET scan revealed strong uptake by the thyroid and lung lesions. In addition, bone metastasis at the left 9th posterior rib and iliac bone were identified by WBBS. The final stages of thyroid and lung cancers were T2N1b and T4N3M1a, respectively.
Fine-needle aspiration cytology of the thyroid lesion was performed, and the result was confirmed as PTC. Cytology via endobronchial ultrasound-guided transbronchial needle aspiration (EBUS-TBNA) revealed an NSCLC, favoring a poorly differentiated adenocarcinoma. Immunohistochemical staining results showed that PD-L1 (SP263) was expressed in 90% of tumor cells, and the L858R EGFR mutation was present.
After the diagnosis of double primary cancers, this case was discussed at the tumor board meeting, and it was decided that the patient would be treated for the lung lesion prior to the thyroid lesion, based on their relative severities.
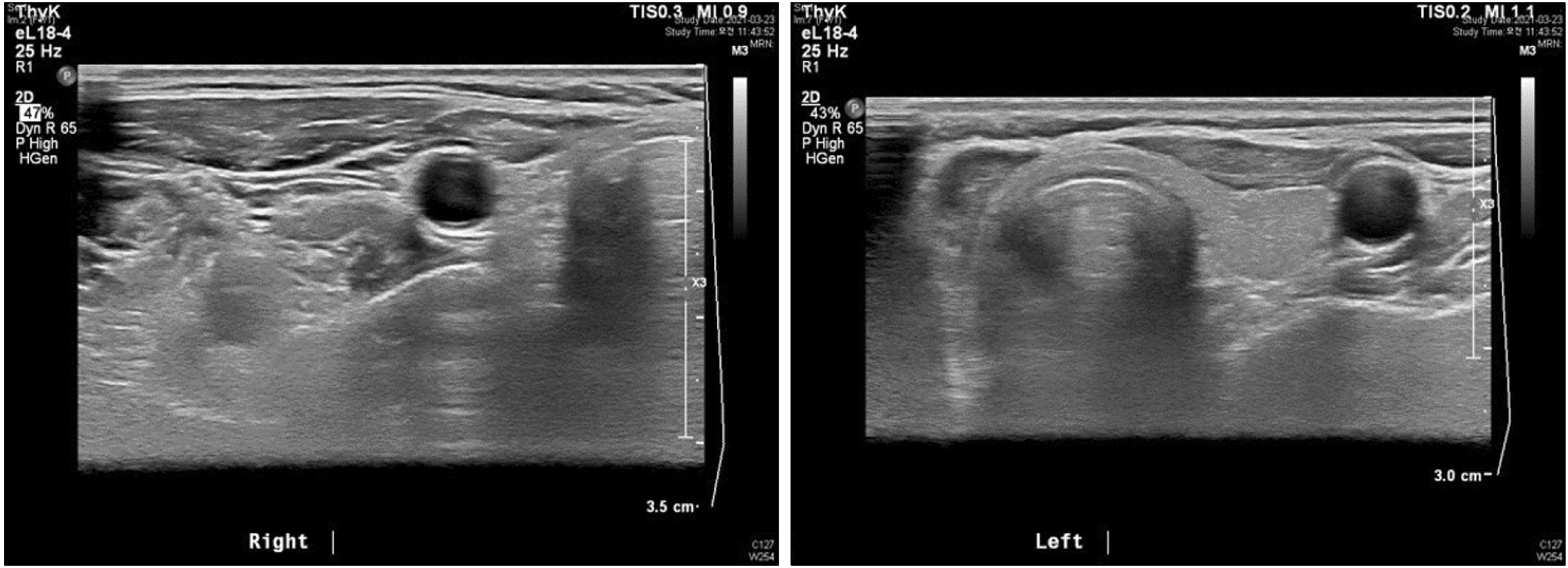
As a first-line treatment for NSCLC with EGFR mutations, the patient started targeted therapy with afatinib (30 mg/day). External radiotherapy for bone metastasis was performed simultaneously. During this period, thyroid lesions were evaluated every 3 months using US and thyroid function tests. After 3 months of afatinib treatment, thyroid US revealed atrophic changes in the right thyroid gland, indicating a post- hemithyroidectomy status (Fig. 2).
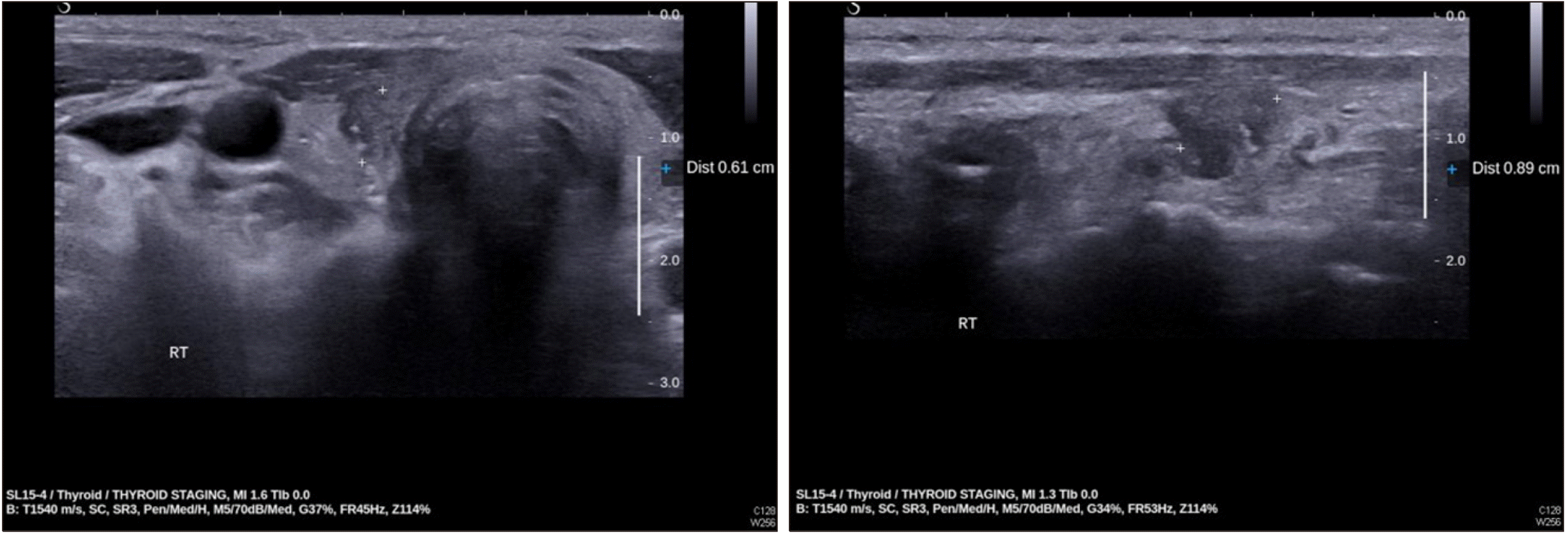
Six months after afatinib therapy, new metastatic bone lesions in the L2 and L4 vertebral bodies and coccyx were noted on spine CT. The treatment regimen was changed to pemetrexed and cisplatin; 6 weeks post new regimen, thyroid US showed an atrophic but visible right thyroid gland and a cancer lesion 0.9 cm in size (Fig. 3).
The patient succumbed to lung cancer progression after 1 month.
Researchers have previously tried to uncover the molecular pathways involved in PTC to identify new molecular targets for treatment. The most important protein kinases differentially expressed in PTC are SRC, MAPK, and EGFR, which could be potential therapeutic targets.6) Some studies have demonstrated that high expression of EGFR in PTC is associated with aggressive behavior, such as extrathyroidal extension or lymph node metastasis.7,8) With these efforts, new drugs have been developed that target known pathways such as rearranged during transfection (RET), BRAF (B-Raf proto-oncogene, serine/threonine kinase), RAS (Rat sarcoma), and vascular endothelial growth factor receptor 2.5)
Afatinib is an orally active, irreversible ErbB-family inhibitor that binds to the kinase domain of EGFR, HER2, and HER4, and is the next generation of EGFR-TKI, erlotinib and gefitinib.3) EGFR inhibition can be used as an initial treatment for NSCLC with EGFR mutations;4) however, a trial reported 48% of patients with differentiated thyroid carcinomas treated with gefitinib 250 mg/day attained stable disease status at 3 months.5) Furthermore, a patient with metastatic thyroid carcinoma with EGFR mutation who responded to erlotinib, has been previously reported.5) These studies demonstrate the potential of EGFR-TKIs, such as afatinib, for use as targeted therapy for PTC with EGFR mutations.
In conclusion, targeted therapy for PTC is not a routine treatment modality. Afatinib is an orally active irreversible ErbB-family inhibitor that binds to the kinase domain of EGFR, HER2, and HER4 and is applied an initial treatment modality for NSCLC with EGFR mutations. Our experience with this case of PTC with EGFR mutation that responded to afatinib suggests that genetic testing might be helpful in considering other treatments for PTC, and EGFR inhibitors may be one of the treatment options for refractory thyroid carcinoma.
References
1. Korea Central Cancer Registry. Annual report of cancer statistics in Korea in 2019. 2021; 2021:66.
2. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2016; 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 26(1):1–133. DOI: 10.1089/thy.2015.0020. PMID: 26462967. PMCID: PMC4739132.

3. Wind S, Schnell D, Ebner T, Freiwald M, Stopfer P. 2017; Clinical pharmacokinetics and pharmacodynamics of afatinib. Clin Pharmacokinet. 56(3):235–50. DOI: 10.1007/s40262-016-0440-1. PMID: 27470518. PMCID: PMC5315738.

4. Keating GM. 2014; Afatinib: a review of its use in the treatment of advanced non-small cell lung cancer. Drugs. 74(2):207–21. DOI: 10.1007/s40265-013-0170-8. PMID: 24435321.

5. Fallahi P, Mazzi V, Vita R, Ferrari SM, Materazzi G, Galleri D, et al. 2015; New therapies for dedifferentiated papillary thyroid cancer. Int J Mol Sci. 16(3):6153–82. DOI: 10.3390/ijms16036153. PMID: 25789503. PMCID: PMC4394525.

6. Zhang H, Gao B, Shi B. 2016; Identification of differentially expressed kinase and screening potential anticancer drugs in papillary thyroid carcinoma. Dis Markers. 2016:2832980. DOI: 10.1155/2016/2832980. PMID: 27703281. PMCID: PMC5040815.

7. Dai Y-J, Qiu Y-B, Jiang R, Xu M, Zhao L, Chen GG, et al. 2017; Concomitant high expression of ERα36, EGFR and HER2 is associated with aggressive behaviors of papillary thyroid carcinomas. Sci Rep. 7(1):12279. DOI: 10.1038/s41598-017-12478-1. PMID: 28947799. PMCID: PMC5612999.

8. Tang C, Yang L, Wang N, Li L, Xu M, Chen GG, et al. 2014; High expression of GPER1, EGFR and CXCR1 is associated with lymph node metastasis in papillary thyroid carcinoma. Int J Clin Exp Pathol. 7(6):3213–23.
Fig. 1
Neck US showing an infiltrative enhancing lesion in the right thyroid gland (A, B), a suspicious meta-static lateral neck lymph node (C, D), and PET-CT demonstrating that right thyroid gland and lung lesion show a strong FDG uptake (E-G).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download